Why wait? The data show administering steroids to DME patients with good vision leads to long-term success
A review of the phase 4 PALADIN data show that steroid-eluting implants lead to good vision, safe outcomes, and reduced burden in patients with DME.
(Image credit: AdobeStock/Jo Panuwat D)

Retina specialists who treat patients with diabetic macular edema (DME) are tasked with striking a delicate balance. The maximizing therapeutic potential while reducing treatment burden and keeping patients as safe as possible may seem like competing directives—and indeed they are to retina specialists who only leverage the benefits of regular intravitreal injections of anti-VEGF agents or local steroids rather than long-term treatment options.
Retina specialists who have embraced steroid-eluting technology for the treatment of DME have seen in their patients improved vision, good safety, and reduced treatment burden. Still, for many years, retina specialists were reluctant to move patients with DME to a long-term steroid strategy, in part because of concerns regarding increased risk of cataract in phakic patients and possible IOP elevations in all patients.1,2
However, an approach averse to steroid-eluting implants overlooks 2 important issues. Repeated office visits for treatment may be disruptive to patients with DME who are employed or are unable to find transportation to the clinic. Further, the more visits on a patient’s calendar, the more likely they are to miss an appointment, which in turn may lead to worse outcomes over time. For some patients, an overly cautious treatment strategy centered on anti-VEGF monotherapy could be counterproductive.
Further, an anti-VEGF–only approach has proven ineffective for a significant number of patients. Patients with DME in the DRCR Retina Network Protocol T study who were regularly dosed with anti-VEGF showed rates of persistent DME as high as 67% at 24 weeks; among those patients, more than two-thirds of them had persistent DME at 2 years.3
Use of steroid-eluting intravitreal implants such as the 0.19-mg fluocinolone acetonide (FAc) implant (Iluvien, Alimera Sciences) or the 0.7-mg dexamethasone implant (DEX; Ozurdex, Allergan) reduces treatment burden in patients, empowers providers to exercise continued control over a patient’s disease, and results in improved outcomes in many patients. In my clinic, I move patients with DME to the 0.19-mg FAc implant (which elutes the drug for up to 36 months) after a patient has demonstrated a safe and effective response to a 0.7-mg DEX implant (which typically lasts 3 to 6 months). As we have learned more about the value of steroid-eluting implants, I have reduced the interval between patient presentation and the time to implant dosing, meaning that patients with DME in my clinic who are good candidates for steroid therapy receive it sooner than they used to.
Data from the phase 4 PALADIN study (NCT02424019) support the use of 0.19-mg FAc in patients with DME who present to the clinic early in their disease course and/or with good functional vision. In PALADIN, patients with good vision (≥ 20/40) were more likely to maintain good vision, experience reduced treatment burden, and experience fewer IOP-related events compared with patients with poor vision (< 20/40).4 Let’s explore thePALADIN study and a recent analysis that examined patients based on presenting vision.
PALADIN
The PALADIN study was a 36-month, nonrandomized, open-label, observational phase 4 study to assess 0.19-mg FAc in 202 eyes in 159 patients with DME.5 All study patients previously received corticosteroid treatment for DME and had not demonstrated a clinically significant rise in IOP.
At 36 months among all patients in the study, significant mean central subfield thickness reductions were observed, as were significant best-corrected visual acuity (BCVA) gains from baseline.5 Treatment burden was meaningfully reduced by 70.5% (3.4 treatments/year in the 36 months before FAc implantation vs 1.0 treatments/year during the 36-month PALADIN study). Among eyes that reached 36 months of treatment, 26% of patients were rescue free at the end of PALADIN. An excellent safety profile was observed, with IOP increases greater than 30 mm Hg occurring in 11% of eyes.5 IOP-related surgery occurred in 3 of those eyes; it was determined that neovascular glaucoma was the cause of IOP elevation.5
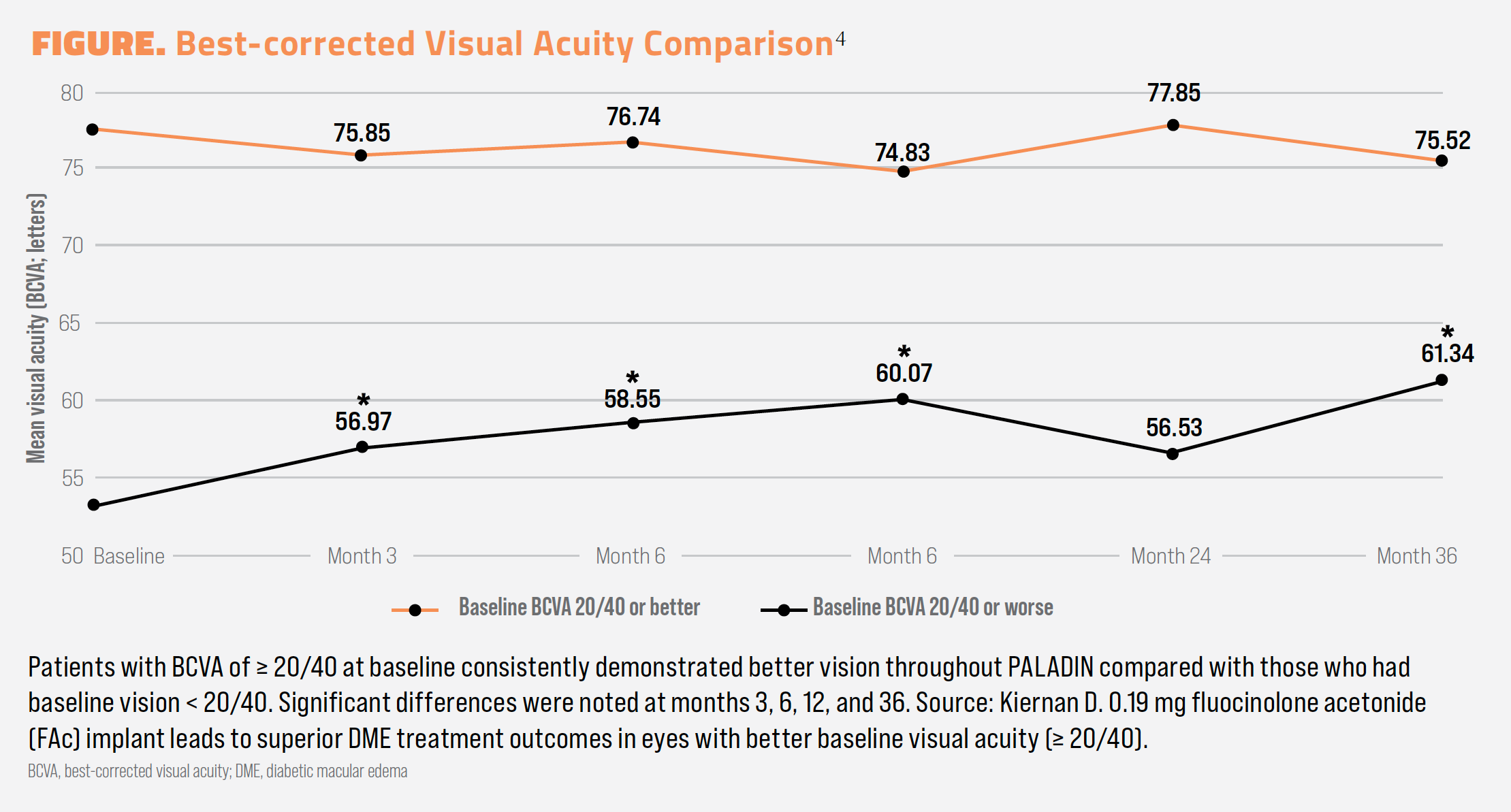
A subanalysis of PALADIN examined outcomes of patients who presented with good vision (> 20/40) or poor vision (< 20/40) at baseline.4 Vision remained stable at 36 months among those with good baseline vision, and visual gains were realized among those with poor vision at baseline (–2.0 letters vs +8.2 letters, P < .05).4 In terms of real vision, those with good vision and poor vision, respectively, had BCVA measured at 75.52 and 61.34 letters at month 36, a difference of approximately 15 letters, which was statistically significant (Figure).4
Safety outcomes also favored the good-vision group.4 Of the 8 patients who underwent incisional glaucoma surgery to address IOP elevations, 6 of them had poor vision at baseline. Among good-vision patients, 23.08% needed IOP-lowering medication compared with 33.33% of patients in the poor-vision group. A fuller assessment of safety can be found in the Table.
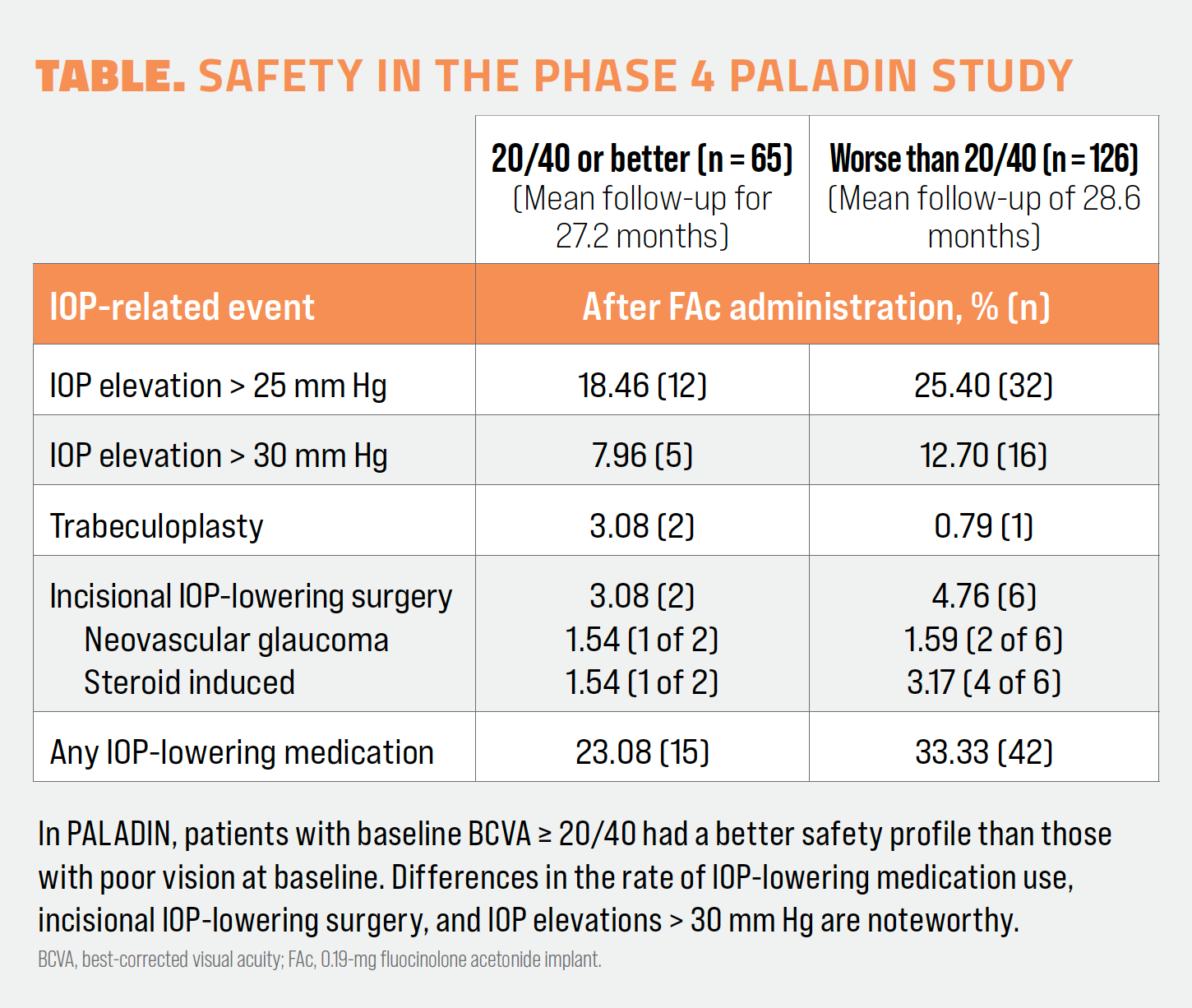
Treatment burden reductions were realized at higher rates in the good-vision group.4 In the group with 20/40 or better vision at baseline, 73% of patients needed 0 to 2 follow-up treatments per year in the 36 months following FAc injection, whereas in the group with worse than 20/40 vision, 63% of patients needed 0 to 2 follow-up treatments per year. More patients in the poor-vision group needed 3 to 5 treatments during the course of the study compared with the good-vision group (28% vs 18%, respectively).
What do these data mean for my practice?
Data from both the overall PALADIN results and the analysis of patients based on baseline VA demonstrate that treatment with the FAc implant is safe and effective in patients with DME, and that patients with 20/40 or better vision experienced better overall vision, good safety, and lighter treatment burden at 36 months compared with patients who had worse than 20/40 vision.
In the clinic, these data point to 2 conclusions: Patients with DME who have shown a response to steroid therapy may be good candidates for a long-duration steroid-eluting implant, and patients with good baseline vision should be considered candidates for the 0.19-mg FAc implant. Some retina specialists may wish to consider moving the DEX and FAc implants farther up their algorithm, dosing patients earlier in their disease course than they might have previously done. With consistent steroid dosing while vision remains good, patients may experience better outcomes and providers may maintain better disease control.
The phase 4 NEW DAY study (NCT04469595) is evaluating the safety and efficacy of the FAc implant in treatment-naive patients with DME.6 Data are expected in 2025. Until then, retina specialists should continue to treat patients with DME as they see fit, adjusting their tactics and strategies as they become aware of new data.
Image courtesy of Daniel F. Kiernan, MD, FACS

Daniel F. Kiernan, MD, FACS
Daniel F. Kiernan, MD, FACS, practices at The Eye Associates in Sarasota, Florida.
References:
Munk MR, Somfai GM, de Smet MD, et al. The role of intravitreal corticosteroids in the treatment of DME: predictive oct biomarkers. Int J Mol Sci. 2022;23(14):7585. doi:10.3390/ijms23147585
Chawan-Saad J, Wu M, Wu A, Wu L. Corticosteroids for diabetic macular edema. Taiwan J Ophthalmol. 2019;9(4):233-242. doi:10.4103/tjo.tjo_68_19
Bressler NM, Beaulieu WT, Glassman AR, et al; Diabetic Retinopathy Clinical Research Network. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136(3):257-269. doi:10.1001/jamaophthalmol.2017.6565
Kiernan D. 01.29 mg fluocinolone acetonide (FAc) implant leads to superior DME treatment outcomes in eyes with better baseline visual acuity (≥20/40). Poster presented at: American Academy of Ophthalmology 2023 annual meeting; November 3-6, 2023; San Francisco, CA.
Singer MA, Sheth V, Mansour SE, Coughlin B, Gonzalez VH. Three-year safety and efficacy of the 0.19-mg fluocinolone acetonide intravitreal implant for diabetic macular edema: the PALADIN study. Ophthalmology. 2022;129(6):605-613. doi:10.1016/j.ophtha.2022.01.015
A study of intravitreal ILUVIEN implant as baseline therapy in patients with early diabetic macular edema (DME) (NEW DAY). ClinicalTrials.gov. Updated June 26, 2023. Accessed February 1, 2024. https://clinicaltrials.gov/study/NCT04469595

