
Behind the scenes of building impactful educational platforms in retina care

Behind the scenes of building impactful educational platforms in retina care

The trial results show promising for the evaluated gene therapy for the treatments of LCA, showing significant vision improvements in pediatric patients.

The characteristics of these inherited retinal diseases make it difficult to define objective, clinically meaningful outcomes that are sensitive enough to measure change over the course of a trial.

Broadwood Partners, who is against the merger, holds approximately 27.5% of the outstanding common stock of STAAR, making them the largest outside stockholder of STAAR.

Christine Kay, MD, presents new findings on MCO-010, a novel optogenetic therapy improving vision in retinitis pigmentosa patients at the Retina Society meeting.

Hear highlights from the Vit-Buckle Society and its presence at EURETINA 2025.

Catch up on this week's highlights in retina.

ISTH0036 demonstrates potential to prevent fibrosis, reduce retinal fluid, and improve vision in AMD and diabetic macular edema patients.

The newly approved technology delivers low-dose microcurrents through closed eyelids to stimulate retinal cells.

EURETINA's 25th anniversary meeting attracts 11,000 participants, showcasing cutting-edge research and fostering global networking among retina specialists.

The submission was based on the safety and efficacy data from the phase 3 (RHODOS) and phase 4 (LEROS) studies.

The window shop provision in the Alcon merger agreement enabled STAAR to accept a competing acquisition proposal and terminate the Alcon merger.

Luxa Biotechnology reveals promising phase 1/2a trial results for RPESC-RPE therapy, showing safety and potential vision restoration in patients with dry AMD.

Conavi Medical advances intravascular imaging with its Novasight Hybrid system, enhancing coronary disease diagnosis and treatment through new technology.

Character Biosciences enhances its leadership team and secures $93 million in Series B funding to advance treatments for degenerative eye diseases.

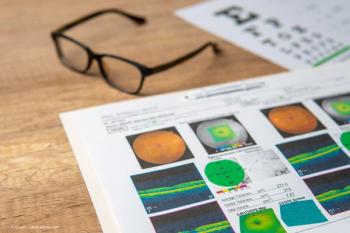
Daniela Bacherini, MD, PhD, FEBO, said modern imaging modalities open up new possibilities in the peripheral retinal space.

Alteogen received a positive CHMP opinion for EYLUXVI in July 2025.

Justis Ehlers discusses promising results from the HELIOS trial, revealing a novel treatment for diabetic retinopathy that may reduce treatment frequency.

Kalaris Therapeutics initiates a phase 1b/2 study for TH103, targeting neovascular age-related macular degeneration and advancing retinal disease treatment.
The country has a goal of ensuring that more than 95% of patients with diabetes receive timely retinal examinations.

Catch up on this week's highlights in retina.

The company’s lead clinical-stage program, OLN324, is a higher potency, higher molar dose VEGF/Ang2 bispecific antibody currently in phase 1b clinical development for patients with either wAMD or DME.

Kodiak Sciences reveals promising APEX study results for KSI-101, showing significant vision improvement in macular edema patients.
The lesion area growth was reduced by more than 50% in the first human clinical trial of K8.

In addition to the proxy statement, STAAR has reached out to all stockholders to ask for their vote to adopt the merger agreement.
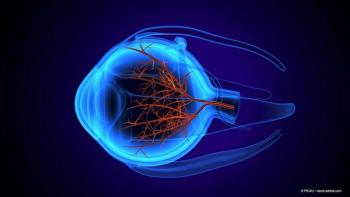
New research reveals significant inner retinal impairment in multiple sclerosis patients without optic neuritis, highlighting the need for further studies on macular health.

Belite Bio completes its phase 3 trial for tinlarebant, a potential first treatment for Stargardt disease, with results expected in late 2025.
Kwangdong, a top 5 pharmaceutical and healthcare company in Korea, is actively involved in research and development innovation, including “transformational late-stage, high-impact technologies.”

Optain Health secures $26 million in Series A funding to enhance AI-driven retinal disease detection and expand its technology.