Clinical trial outcomes for macular edema don’t translate to ‘real-world’ settings
Patents gained less vision in clinical practice than patients on clinical trials

Patients with macular edema from retinal vein occlusions (RVO) treated with anti-VEGF injections in the real-world setting have worse visual outcomes than their clinical trial counterparts.
These outcomes seem to be tied to the number of injections a patient receives in the first year and further illustrate the long-held belief among retinal specialists that clinical trial outcomes do not always translate to clinical practice.
Related: ASRS 2020: Diabetic retinopathy progression in anti-VEGF versus FA implant
Thomas A. Ciulla, MD, MBA, Indiana University School of Medicine, presented the results of “Visual Acuity Outcomes and Anti-Vascular Endothelial Growth Factor Therapy Intensity in Macular Edema Due to Retinal Vein Occlusion: A Real-World Analysis of 12,214 Eyes” as part of ARVO 2020).[TAC1]
The study was a retrospective analysis of 12,214 eyes from the Vestrum Health Retina Database.
The database included electronic medical records from hundreds of retinal specialists across the United States, aggregated and deidentified.
To be eligible for the retrospective review, patients had to have treatment-naive RVO-related macular edema with no other retinal diagnoses, be treated with at least one anti-VEGF injection between 2013 and 2019, and have at least 1 year of follow-up.
Eligible patients were separated into two groups: those with central retinal vein occlusion (CRVO)-related macular edema (5,300 eyes) and those with branch retinal vein occlusion (BRVO)-related macular edema (6,914 eyes).
Related: New Protocol V findings offer guidance for managing DME
Mean age in both groups was 72, and approximately half of the patients were female. Mean baseline visual acuity was 20/80 (56 letters) and 20/160 (39 letters) in the BRVO and CRVO groups, respectively.
At 1 year of follow-up, patients in both real-world groups had an average approximating 7 anti-VEGF injections, with a small percentage of patients receiving additional therapies such as intravitreal steroids (9%, both groups), focal laser treatment (12% BRVO vs 2% CRVO), and panretinal laser treatment (3% BRVO vs 6% CRVO).
In the real-world setting, patients with BRVO and CRVO gained 8.1 and 7.1 letters, respectively, after 1 year of treatment.
However, in registration trials, patients with BRVO and CRVO gained 18 and 16 letters, respectively.
“This difference between real-world study results and registration trial results represents approximately 10 letters on nearly 2 lines of vision,” Dr. Ciulla said.
Related: Improving patient comfort with intravitreal injections
The number of injections a patient receives in the first year seems to directly correlate with visual gains (Figure 1).
For example, patients in the real-world who received 9 or more injections in 1 year generally gained more than 2 lines of vision.
Figure 1. Number of Injections Correlates to Visual Gains. Abbreviations: VA, visual acuity; BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion
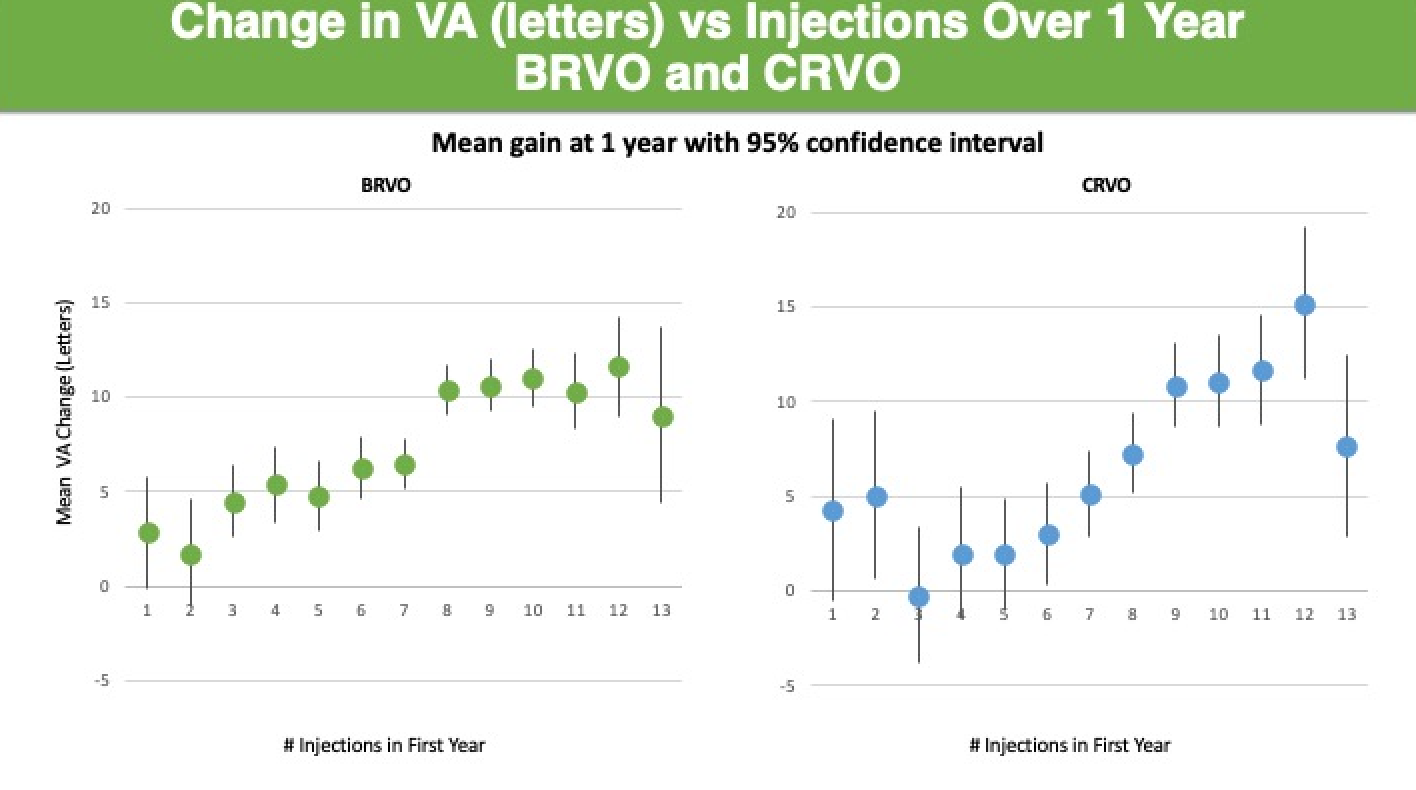
“For both BRVO- and CRVO-related macular edema, mean change in visual acuity over 1 year increased with mean number of injections,” Dr. Ciulla said.
Paradoxically, patients with a baseline visual acuity of 20/40 or better tended to lose vision after 1 year.
“Those with worst baseline visual acuity showed the largest gain in visual acuity over 1 year, while those with the best baseline visual acuity were at the greatest risk of vision loss.This is consistent with an expected ceiling effect.” Dr. Ciulla said.
In terms of number of injections and letter gains, it is instructive to compare these results to real-world outcomes for anti-VEGF therapy in patients with neovascular age-related macular degeneration (>49,000 eyes; 7.3 anti-VEGF injections per eye; +1 letter at 1 year) and diabetic macular edema (>28,000 eyes; 6.4 anti-VEGF injections per eye; +4.2 letters at 1 year) when pulling from the same database. 1,2
Related: How DME patients may fare differently in ‘real world’ versus trials
“Overall, real-world RVO-related macular edema patients experienced greater 1-year vision gain than a real-world patient with age-related macular degeneration and diabetic macular edema, but exhibit a larger gap in visual gain when compared to respective randomized controlled trials,” he said.
References
1. Ciulla TA, Hussain RM, Pollack JS, Williams DF, Visual Acuity Outcomes and Anti-Vascular Endothelial Growth Factor Therapy Intensity in Neovascular Age-Related Macular Degeneration Patients: A Real-World Analysis of 49 485 Eyes. Ophthalmol Retina, 2020. 4(1): p. 19-30.
2. Ciulla TA, Pollack JS, Williams DF, Visual acuity outcomes and anti-VEGF therapy intensity in diabetic macular oedema: a real-world analysis of 28 658 patient eyes. Br J Ophthalmol, 2020. Online ahead of print. https://bjo.bmj.com/content/bjophthalmol/early/2020/04/07/bjophthalmol-2020-315933.full.pdf
3. Ciulla TA. Visual Acuity Outcomes and Anti-Vascular Endothelial Growth Factor Therapy Intensity in Macular Edema Due to Retinal Vein Occlusion: A Real World Analysis of 12,214 Eyes. Invest Ophthalmol Vis Sci. 2020 Jun. 61: 3523.
Newsletter
Keep your retina practice on the forefront—subscribe for expert analysis and emerging trends in retinal disease management.