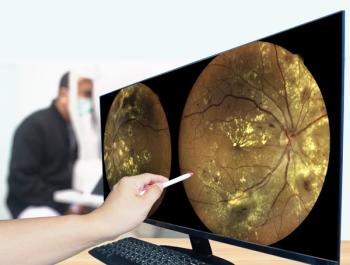
DME: What it is and how to treat it
The Centers for Disease Control estimates that more than 84 million U.S. adults, or 40% of the U.S. adult population, is prediabetic. According to the recent Prevent Blindness study, “Future of Vision: Forecasting the Prevalence and Costs of Vision Problems,” there are more than 8.1 million Americans with diabetic retinopathy. The projected total cases of diabetic retinopathy will increase by 35% to 10.9 million by 2032, and by 63% to 13.2 million by 2050.
Risk factors for diabetic retinopathy and diabetic macular edema (DME) include all types of diabetes, but women with diabetes who are pregnant and people who have had diabetes for long periods are at particular risk. In fact, as the duration of diabetes lengthens, it becomes not a matter of if a patient will develop diabetic retinopathy but a question of how much. Approximately one-third of patients with diabetes have some stage of diabetic retinopathy, and only half of these patients are aware of it. Diabetic retinopathy is asymptomatic in early stages; therefore, it is critical that patients with diabetes receive routine eye exams to check for and monitor diabetic retinopathy and DME to prevent disease progression.1
However, despite the known necessity of eye exams in patients with diabetes, the American Academy of Ophthalmology (AAO) has found that only 60% of diabetics have annual screenings for diabetic retinopathy and DME.2 The AAO recommends that patients with Type 1 diabetes begin annual screenings 5 years after disease onset, whereas patients with Type 2 diabetes must have an immediate screening at the time of diagnosis and comprehensive eye exams every year or every 6 months. Physicians must impress upon patients the relentlessness of the disease; just because the patient doesn’t have diabetic retinopathy and/or DME currently doesn’t mean it won’t develop.
Screening for DME and diabetic retinopathy should include optical coherence tomography (OCT) imaging, repeat angiograms, and widefield imaging. OCT-A should be included when available. DME can be prevented, and progression can cease, with proper glucose and blood pressure control. The AAO recommends that physicians routinely remind patients the importance and overall health benefits of maintaining near-normal glucose levels and blood pressure. A diagnosis of diabetic retinopathy and/or DME should be communicated to the patient’s primary care physician.
Treatment for diabetic retinopathy and DME pose many challenges for physicians and patients. Treatments include anti-vascular endothelial growth factor (VEGF) injections with agents such as bevacizumab (Avastin, Genentech), ranibizumab (Lucentis, Genentech), and aflibercept (Eylea, Regeneron), which require patients to have medications injected directly into their eyes on a routine basis; laser surgery; and corticosteroid implants, which can be used in combination with other treatments.
Treatment success is heavily reliant on patient compliance and buy-in because treatment requires a long-term commitment. Anti-VEGF injections are needed fairly regularly, especially in the early treatment phases, leading to a significant injection burden for physicians and patients alike. The disease course is unpredictable; therefore, it is difficult to set expectations with patients on the number of injections and treatments needed to control disease progression. Because of these and other factors, noncompliance and loss to follow-up are common in patients with DME and diabetic retinopathy.3
However, clinical trials have shown that successful treatment outcomes are possible when patients are compliant. For example, the Early Treatment Diabetic Retinopathy Study found that laser treatment reduced vision loss from DME by 50%.4 Protocol T, which compared the safety and efficacy of aflibercept, bevacizumab, and ranibizumab for DME treatment, found that all three agents showed improvements in visual acuity after 3 to 6 months of consecutive monthly injections when baseline visual acuity loss was mild (20/32 to 20/40).5 Anti-VEGF agents are proven to be effective treatments in patients with DME if routine injections are given.
Clinical trials are currently ongoing to further advance the treatment of DME and diabetic retinopathy. The Diabetic Retinopathy Clinical Research Network (DRCR.net), which was founded in 2002 and funded by the National Eye Institute, is facilitating a number of multicenter clinical trials in diabetic retinopathy and DME. The DRCR.net has significantly contributed to research in these diseases through 25 protocols that are either published, enrolling, or in follow-up.
References:
1. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556-64.
2. American Academy of Ophthalmology. Diabetic Retinopathy Preferred Practice Patterns. 2017; v. 2018.
3. Ehlken C, Helms M, Bohringer D, et al. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2018;12:13-20.
4. Relhan N, Flynn HW, Jr. The Early Treatment Diabetic Retinopathy Study historical review and relevance to today's management of diabetic macular edema. Curr Opin Ophthalmol. 2017;28:205-12.
5. Cai S, Bressler NM. Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: recent clinically relevant findings from DRCR.net Protocol T. Curr Opin Ophthalmol. 2017;28:636-43.
Newsletter
Keep your retina practice on the forefront—subscribe for expert analysis and emerging trends in retinal disease management.