FDA approves generic solution of cyclosporine ophthalmic emulsion for dry eye
The FDA has approved the first generic of cyclosporine ophthalmic emulsion (Restasis; Allergan) 0.05% single-use vials of eye drops to increase tear production in patients with dry eye disease.
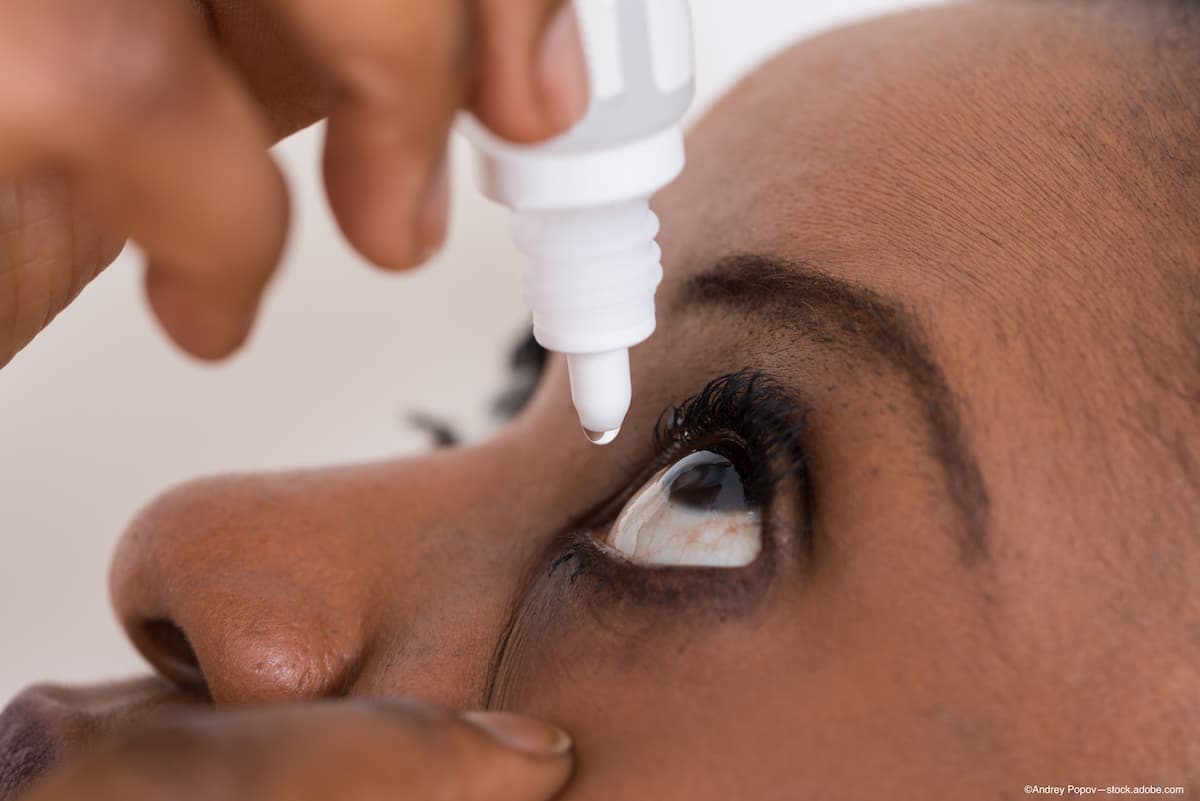
The FDA has approved the first generic of cyclosporine ophthalmic emulsion (Restasis; Allergan) 0.05% single-use vials of eye drops to increase tear production in patients with dry eye disease.
According to a news release, tear production was not seen in patients currently taking topical anti-inflammatory drugs or using punctal plugs.
The generic version is sponsored by Mylan Pharmaceuticals. The company is part of Viatris.
Sally Choe, PhD, director of the Office of Generic Drugs in FDA’s Center for Drug Evaluation and Research, pointed out in the news release that restasis has been approved for use in the U.S. for nearly 20 years, but until today, there was no approved generic product of this drug.
“Today’s approval reflects the FDA’s continued commitment to advancing patient access to lower-cost, high-quality generic drug products that are as safe and effective as their brand name counterparts,” she said in a statement. “Supporting development and expanding opportunities to bring complex generic drugs to the market is a major focus of our efforts to help improve competition and help lower drug prices.”
Dry eye occurs when a person’s eyes don’t make enough tears to stay wet, or when the tears are not of the correct consistency. This condition, affecting millions of Americans each year, can make the eyes feel uncomfortable.
Cyclosporine ophthalmic emulsion is a prescribed immunomodulator (affects the functioning of the immune system) with anti-inflammatory effects that generally helps to increase tear production in these patients.
The most common side effect reported in the clinical trials for cyclosporine ophthalmic emulsion was ocular burning. Other reactions included conjunctival hyperemia (dilation and redness of blood vessels in the eye), discharge, epiphora (excessive watering of the eye), eye pain, foreign body sensation (the sensation of having something in your eye), pruritus (itchy skin), stinging and visual disturbance (most often blurring).
According to the FDA, applicants must submit appropriate data and information to demonstrate that generic drug products meet the FDA’s rigorous approval standards, ensuring that generic drug products are safe, effective and meet the same high-quality standards as their brand name counterparts.
The development of complex generics may be more difficult due to, for example, their complex active ingredient formulation or route of delivery. As a result, many complex drugs lack generic competition. The FDA has taken a multifaceted approach to encourage development of complex generics through the Generic Drug User Fee Amendments (GDUFA) program.
Newsletter
Keep your retina practice on the forefront—subscribe for expert analysis and emerging trends in retinal disease management.