Gene variants point to a potential cause of age-related macular degeneration
According to National Eye Institute researchers, the variants generate malformed proteins that alter the stability of the membrane attack complex, which may drive a chronic inflammatory response in the retina.
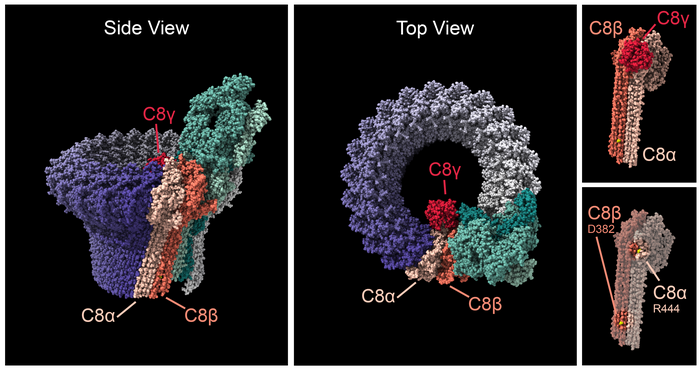
The complement membrane attack complex is a pore that inserts into the cell membrane. The complex is formed by up to 18 C9 subunits (purple), the C5, C6, and C7 subunits (various shades of green), and the C8-alpha, C8-beta, and C8-gamma subunits (shades of red). Side (left) and Top (center) views show the complex's split-washer configuration. C8 subunits bind the C9 ring with the remainder of the complex. Ultra-rare mutations (right, depicted in yellow) in C8-alpha at arginine 444 and in C8-beta at residue aspartic acid 382 appear to either stabilize or destabilize the membrane attack complex. Changes in the stability of the membrane attack complex may lead to chronic inflammation of the retina. (Image courtesy of National Eye Institute/National Institutes of Health)
A study from the National Eye Institute (NEI) identified rare genetic variants that could point to one of the general mechanisms driving age-related macular degeneration (AMD), a common cause of vision loss in older adults. The variants generate malformed proteins that alter the stability of the membrane attack complex (MAC), which may drive a chronic inflammatory response in the retina.
The findings, published in the journal iScience,1 point to MAC as a potential therapeutic target to slow or prevent the development of AMD. NEI is part of the National Institutes of Health.
According to an NEI news release, there are many known genetic variants that raise or lower an individual’s risk of getting AMD; however, the contribution of each of these genetic changes to AMD is small.
To discover genetic variants – and proteins – with a direct tie to the disease, Anand Swaroop, PhD, chief of NEI’s Neurobiology, Neurodegeneration and Repair Laboratory, and lead author of the study, undertook a collaboration with Michael Klein, MD, a leading AMD clinician at the Oregon Health Sciences University (OHSU), Portland.
According to NEI, Klein collected clinical information for hundreds of patients, as well as families with a high number of individuals with AMD. Swaroop, Klein and colleagues looked for families carrying very rare AMD-causing variants, where the effect of the gene variant is very strong, and where the variant directly affects protein structure and function. This type of rare variant can reveal the root cause of disease.
Swaroop pointed out that while scientists have known about many genetic variants that affect AMD risk, only a few have pointed directly to protein alterations that can cause AMD.
“By looking at large families with ultra-rare variants that track closely with disease across generations, we found two proteins that may directly be the driving force behind AMD pathology in affected patients,” Swaroop. “These proteins could be targets for future drugs.” While there are currently some treatments to slow vision loss for people with the wet form of AMD, there is no treatment for most patients and no cure for the disease.
Swaroop, Klein and colleagues found that in four families, individuals with AMD have mutations in one of two proteins that form one end of MAC: C8-alpha and C8-beta. The team found that the variants from the four AMD families all affected the ability of the C8 proteins to stick to each other, which may alter how MAC behaves in the eye’s retina.
MAC forms a circular pore, closed at one end by the C8 proteins; the MAC pore permits the flow of ions through the outer membrane of cells. This pore is the final step in the ‘complement cascade,’ a part of the immune system that helps the body defend against pathogens. Although scientists initially thought that MAC’s only function was to insert into bacterial cell membranes and kill the pathogen, more recent evidence shows that MAC plays a complex role in regulating inflammatory processes in tissues like the retina.
Genetic data from NEI’s Age Related Eye Disease Studies have suggested roles for C8 proteins, as well as other proteins higher up in the complement cascade, in AMD. Because MAC is the final step in the complement cascade, variants affecting any of the complement proteins may funnel down to alter MAC function. The researchers believe that either too much or too little stable MAC in the retina may lead to destructive inflammation, which in turn drives AMD progression.
“Given that MAC is the end of the immune system’s complement pathway, and because there’s such a strong link between these rare variants and disease, we think that targeting it may be a more effective strategy to control AMD,” Swaroop said. “With a small molecule drug, we might be able to control how strongly MAC drives inflammation, and from there slow down progression of AMD.”
The research was funded by the National Eye Institute, as well as Research to Prevent Blindness, the Retina Research Foundation, and the Casey Eye Institute Macular Degeneration Center.
Reference
1. Zelinger L, Martin TM, Advani J, Campello L, English MA, Kwong A, Weber C, Maykoski J, Sergeev YV, Fariss R, Chew EY, Klein ML, and Swaroop A. “Ultra-rare complement factor 8 coding variants in families with age-related macular degeneration.” iScience. April 1, 2023.
Newsletter
Keep your retina practice on the forefront—subscribe for expert analysis and emerging trends in retinal disease management.
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.