Implementing at-home monitoring for exudative AMD
Investigational OCT system with AI-based image analysis holds promise

A deep learning artificial intelligence algorithm for automated analysis of images from home-based OCT performed comparably to human readers for localizing and quantifying macular fluid in eyes with exudative age-related macular degeneration (AMD), said Judy E. Kim, MD, at the 2020 virtual meeting of the American Society of Retina Specialists.
“Currently, much of our diagnosis and management of eyes with exudative AMD is dependent on OCT. Therefore, at-home diagnostic testing, such as home OCT, offers opportunity for personalizing disease management by providing inter-visit information that allows us to catch progression or recurrence as soon as it happens. As a result, we may be able to reduce both the likelihood of undertreating and the treatment burden while maintaining visual acuity over the course of treatment,” said Kim, professor of ophthalmology, Medical College of Wisconsin, Milwaukee, Wisconsin.
Kim

“This investigational OCT fluid evaluation system can analyze the fluid status and volume dynamics in eyes with exudative AMD and can localize the fluid down to sub-nanoliter volumes. We believe the system has the potential to accurately monitor AMD disease activity in a self-operated home environment, which would be a paradigm shift in patient management. Use of this system might also give us new insights into the markers of wet AMD activity, such as the patterns of fluid distribution between intra- and subretinal fluid, fluid localization in the retina and the changes in fluid volume over time.”
System description
Both the home OCT device (Notal Home OCT, Notal Vision) and deep learning algorithm (Notal OCT Analyzer) are investigational in the United States. The OCT is a compact SD-OCT unit (Figure 1) that can be self-operated by patients. It takes cross-sectional images of the central 10º (3 x 3 mm) of the macula and generates 88 B-scans with dense, 34 µm spacing. The images are uploaded to the cloud for automated analysis by the artificial intelligence-based algorithm.
Figure 1: Compact Home OCT

The algorithm was developed to detect and quantify retinal fluid in exudative AMD and to follow the volume quantities over time. In addition to the home-based OCT device, it can analyze images acquired with commercially available SD-OCT platforms (Cirrus, Carl Zeiss Meditec; Spectralis, Heidelberg Engineering).
The analyzer automatically segments the OCT images and then identifies and quantifies subretinal fluid (SRF) and intraretinal fluid (IRF). The fluid status report generated by the system includes a fluid thickness map that shows the distribution and location of fluid and fluid volume tracking over time.
Accuracy testing
The performance of the deep learning algorithm for identifying and quantifying retinal fluid from the home OCT images was analyzed using data from 355 eyes of 239 patients with AMD. The eyes were randomly split 8:1 into learning and validation datasets. From each volume scan of 88 images, about 10 B-scans were selected for manual segmentation and each B-scan was labeled pixel-wise into four compartments (vitreous/outer layers, retina, SRF, IRF).
A total of 3428 B-scans, of which 75% had fluid, were manually segmented. The performance of the deep learning algorithm for quantification of B-scan segmented fluid was evaluated by comparison to determinations of a human grader, and the results showed excellent agreement (Pearson correlation coefficients = 0.98 for SRF and 0.90 for IRF). Pixel-wise fluid was compared with recall/precision. The SRF fluid pixel-wise recall was 0.72 and precision was 0.86; the IRF recall was 0.80 and the precision was 0.77. The system also demonstrated excellent accuracy for detecting SRF and IRF in the B-scans with sensitivity and specificity of 0.99 and 0.98, respectively, for SRF and 0.99 and 0.97, respectively, for IRF.
Case study
In her presentation, Kim illustrated the use of the home-based OCT system for monitoring a patient undergoing anti-VEGF therapy for wet AMD. The patient was an 82-year-old male who was under the care of Anat Loewenstein, MD, Tel Aviv, Israel.
Figure 2: AI-based fluid quantification, mapping and tracking over time in a patient under anti-VEGF therapy (A. Loewenstein, Tel Aviv Medical Center, Tel Aviv, Israel)
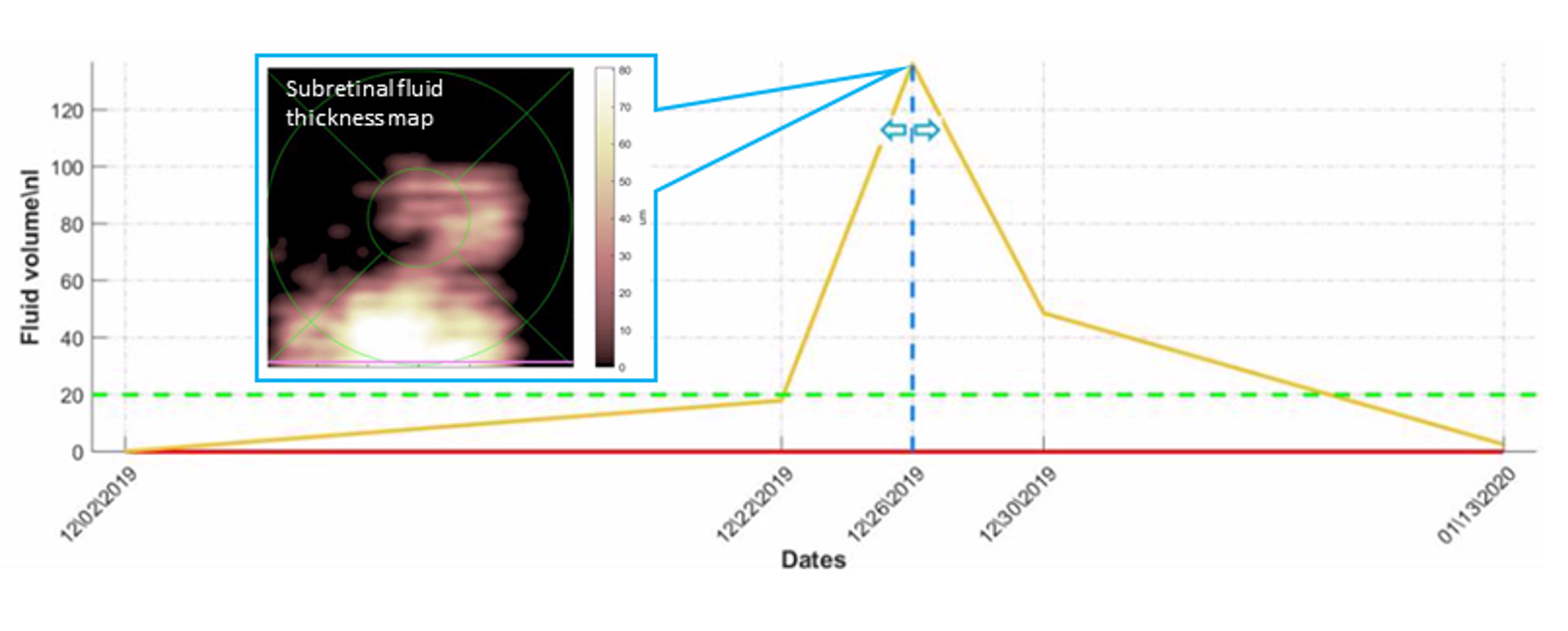
Kim presented reports from images obtained over a period of 42 days from a patient that on an earlier visit had received an anti-VEGF injection (Figure 2). At baseline, the algorithm detected 18 picoliters of fluid in the subretinal space, which was barely visible to the physician. The fluid volume increased to 18 nanoliters on day 20 and more dramatically to 124 nanoliters within the next 4 days. The patient received an intravitreal injection of an anti-VEGF agent on day 24. The fluid volume decreased to 45 nanoliters on day 28 and to just 2.8 nanoliters on day 42.
“Marked improvement after the intravitreal injection was demonstrated on the fluid map as well as in the longitudinal fluid volume graph,” Kim said.
“The artificial intelligence-based analysis of OCT images provides new insights into temporal retinal fluid dynamics.”
Judy E. Kim, MD
E: jekim@mcw.edu
Kim receives research support from and is on the advisory board for Notal Vision.
Newsletter
Keep your retina practice on the forefront—subscribe for expert analysis and emerging trends in retinal disease management.