Loss of "youth" protein may drive aging in the eye, NEI study finds
Eyes of mice lacking protective protein show signs similar to age-related macular degeneration.
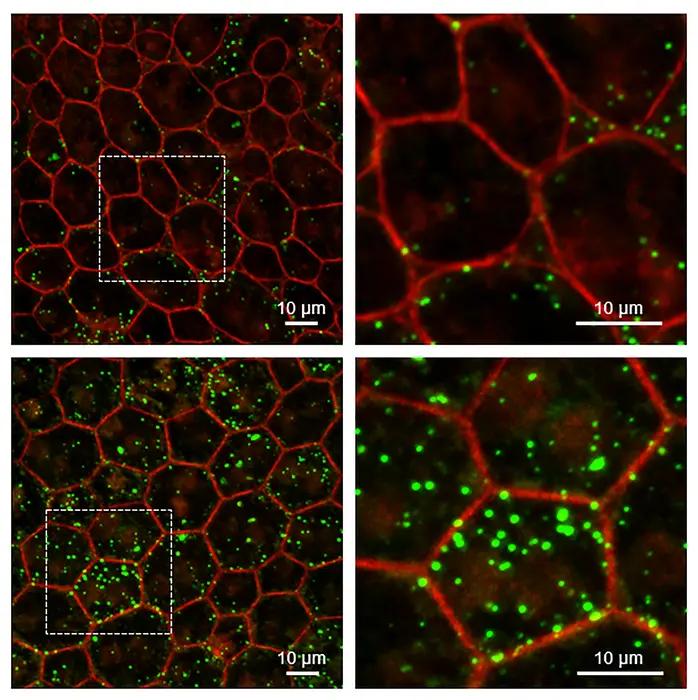
RPE from mice without Serpin1 accumulate more lipids than wild-type mice. Super-resolution confocal microscopy of RPE tissue from wild-type (upper) and Serpin1-null (lower) mice. Detailed images on the right are magnified regions of the RPE tissue imaged on the left (dotted square area). RPE cell boundaries are stained in red, and accumulated lipids are stained in green. (Image courtesy of Ivan Rebustini/NEI)
Loss of the protein pigment epithelium-derived factor (PEDF), which protects retinal support cells, may drive age-related changes in the retina, according to a new study in mice from the National Eye Institute (NEI).
Aging-associated diseases of the retina, like age-related macular degeneration (AMD), are a leading cause of blindness among patients. This new finding could lead to therapies to prevent AMD and other aging conditions of the retina. The study1 was published in the International Journal of Molecular Sciences. NEI is part of the National Institutes of Health.
"People have called PEDF the ‘youth’ protein, because it is abundant in young retinas, but it declines during aging," Patricia Becerra, PhD, chief of NEI’s Section of Protein Structure and Function and senior author of the study, said in a NHI news release. “This study showed for the first time that just removing PEDF leads to a host of gene changes that mimic aging in the retina.”
According to the news release, the retinal pigment epithelium (RPE), a layer of support cells, nourishes photoreceptors and recycles pieces of the photoreceptor cells called "outer segments," which get used up and their tips shed each time photoreceptors detect light. If the RPE cannot provide recycled components of older outer segment tips back to photoreceptors, these cells lose their ability to make new segments, and eventually become unable to sense light. And without nutrients supplied by the RPE, photoreceptors die. In people with AMD or certain types of retinal dystrophies, senescence (aging) or death of RPE cells in the retina leads to vision loss.
The NIH noted that past work from Becerra’s lab and others has shown that PEDF protects retinal cells, preventing both damage to the cells and abnormal growth of blood vessels in the retina. RPE cells produce and secrete the PEDF protein. The protein then binds to its receptor, PEDF-R, which is also expressed by RPE cells. Binding by PEDF stimulates PEDF-R to break down lipid molecules, key components of the cell membranes that enclose photoreceptor outer segments and other cellular compartments. This breakdown step is a key part of the outer segment recycling process. And while researchers have known that PEDF levels drop in the retina during the aging process, it was not clear whether this loss of PEDF was causing, or merely correlated with, age-related changes in the retina.
To examine the retinal role of PEDF, Becerra and colleagues studied a mouse model that lacks the PEDF gene (Serpin1). The researchers examined the cellular structure of the retina in the mouse model, finding that the RPE cell nuclei were enlarged, which may indicate changes in how the cells’ DNA is packed. The RPE cells also had turned on four genes associated with aging and cellular senescence, and levels of the PEDF receptor were significantly below normal. Finally, unprocessed lipids and other photoreceptor outer segment components had accumulated in the RPE layer of the retina. Similar changes in gene expression and defects in RPE metabolism are found in the aging retina.
"One of the most striking things was this reduction in the PEDF receptor on the surface of the RPE cells in the mouse lacking the PEDF protein," the study’s lead author, Ivan Rebustini, PhD, a staff scientist in Becerra’s lab, said in the news release. "It seems there’s some sort of feedback-loop involving PEDF that maintains the levels of PEDF-R and lipid metabolism in the RPE."
While at first glance, the retinas of these PEDF-negative mice appear normal, these new findings suggest that PEDF is playing a protective role that helps the retina weather trauma and aging-related wear and tear.
"We always wondered if loss of PEDF was driven by aging, or was driving aging," Becerra said in the news release. "This study, especially with the clear link to altered lipid metabolism and gene expression, indicates the loss of PEDF is a driver of aging-related changes in the retina."
The study was supported by the NEI intramural program.