Monitoring patients for neovascular AMD in the office and at home
Technology that allows consistent monitoring could be useful tools in 2022—and may be the key to tracking nAMD progression in fellow eyes among patients who have had intervals extended due to new technology.

The fixed, monthly dosing regimens used in pivotal trials evaluating the safety and efficacy of anti-VEGF agents for the treatment of neovascular AMD (nAMD) proved to be impractical in real-world settings. The growing adoption of spectral-domain optical coherence tomography (SD-OCT) at the time of anti-VEGF agents’ approvals enabled anatomically driven, personalized dosing patterns in the form of as-needed and treat-and-extend (TAE) regimens.
Despite our efforts, the real-world visual outcomes of anti-VEGF treatment have fallen short of those seen in pivotal trials, a trend driven primarily by undertreatment. The retina community must recognize that office-based diagnostics provide mere snapshots in time of a highly dynamic and heterogeneous disease. Moreover, anatomic endpoints measured on OCT such as central retinal thickness, which were established to describe disease activity, have borne limited correlation to visual acuity outcomes. Given the significant cost of treatment and the small percentage of patients who maintain functional vision after several years of anti-VEGF therapy, our field must look for new solutions to manage our patients.
Moving beyond the Amsler Grid
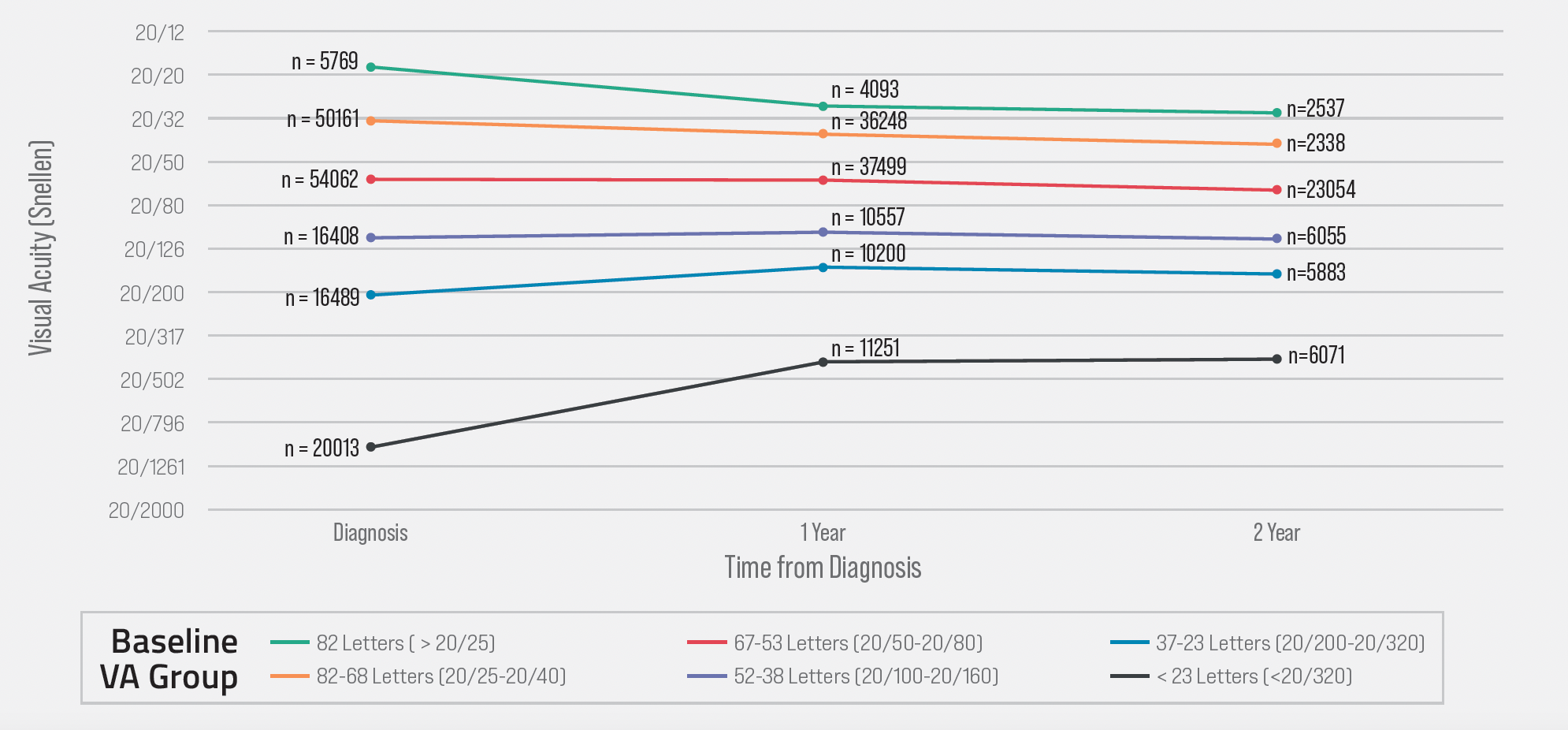
Long-term treatment outcomes are largely predetermined by a patient’s visual acuity at the time of nAMD diagnosis. Reliance on infrequent routine office visits and self-reported symptoms means that only 36% of nAMD patients present at the time of diagnosis with at least 20/40 VA, per real-world IRIS Registry data.1 A subgroup analysis of that dataset found that eyes with 20/40 baseline vision maintain functional vision at 2 years, and that those eyes with presenting VA of <20/40 never reach 20/40 VA after 2 years of treatment (Figure 1). Early detection of conversion from intermediate AMD to nAMD requires that we expand our armamentarium beyond the Amsler grid.
Figure 1. Baseline visual acuity (VA) predicts long-term outcomes of anti-VEGF therapy as shown in Intelligent Research in Sight Registry data analysis by baseline VA group.
An artificial intelligence-enabled, at-home patient monitoring system that has proven to be effective in both randomized controlled clinical trials and in real-world settings utilizes the ForeseeHome preferential hyperacuity perimeter to detect metamorphoptic changes caused by nAMD conversion.2 In a recent analysis of almost 9,000 real-world patients who used such a monitoring system, 81% of conversions to nAMD were caught at visual acuity of 20/40 or better.3
If monitoring systems similar to the one reviewed in this study were to be widely adopted, a higher percentage of patients with 20/40 VA may present to the clinic at the time of nAMD diagnosis. In such a system, office-based eye care providers would prescribe home monitoring for patients at risk of converting to nAMD, and a dedicated remote monitoring service would support the patient in their monitoring journey, thereby forming a new ecosystem of support for early disease detection.
Remote monitoring service providers can become important partners in these emerging decentralized care models by fronting the investment for a large fleet of securely tele-connected home-use devices, training patients on using home monitoring platforms, and tracking patient compliance. Medicare coverage for the ForeseeHome program in the United States has allowed the Notal Vision Monitoring Center to become the first ophthalmic remote monitoring service to establish a patient engagement infrastructure of clinically trained ophthalmic professionals. It should be noted that the trained specialists at the Notal Vision Monitoring Center have replaced the non-specialized call center staff utilized in earlier iterations of the program.
Monitoring for the modern treatment era
Changes in treatment options require us to rethink monitoring and diagnostic approaches to nAMD. Take the expected uptake of the Port Delivery System with Ranibizumab (PDS; Susvimo) as an example. The PDS aims to reduce the number of office visits for patients with nAMD who required frequent treatment. Typically, patients with unilateral nAMD would have their fellow eye monitored for conversion to nAMD each visit. However, given the reduced refill frequency of patients implanted with the PDS, fellow eyes with intermediate AMD are at increased risk of undetected conversion to nAMD. In these patients, a monitoring scheme that includes the ForeseeHome platform may help detect conversion to nAMD when visual acuity is still above the 20/40 threshold, thereby increasing the likelihood that long-term visual function will exist in that eye following therapy.
Patients who are already undergoing anti-VEGF therapy for the treatment of nAMD may also benefit from at-home technology—particularly if they are undergoing TAE regimens that rely on anatomic endpoints to determine whether therapy should be administered. Home OCT systems have shown promise in early clinical trials. Successful patient self-operation of the Notal Vision Home OCT system was reported in 93% of eyes with visual acuity as low as 20/320.4 The system uses a deep learning–based algorithm for automated, quantitative evaluation of OCT scans. Longitudinal data from studies in Israel and the United States have demonstrated high agreement with human expert grading for the presence and quantity of retinal fluid, and imaging reports have allowed detailed characterization of fluid dynamics.5
Some patients receiving anti-VEGF treatment for nAMD on TAE regimens have demonstrated fluid accumulation during the interval between visits (Figure 2). Use of home OCT could alleviate real-world vision loss in these patients by providing alerts when a patient’s fluid volumes exceed a tolerable level, thus allowing proactive, personalized treatment based on anatomic findings. Specific thresholds for intra- and subretinal volumes will allow highly individualized fluid management.
Figure 2. Artificial intelligence-based quantification of intraretinal and subretinal fluid volume can be calculated following frequent home optical coherence tomography imaging. Here, fluid exposure prior to prescheduled treat-and-extend office visits show cause for concern.

Patient compliance with self-imaging needs to be high for the goal of minimizing cumulative fluid exposure to be achieved. Heier and Holekamp recently reported findings from a feasibility study which found that at lease 1 eye was scanned 5.7 days per week among patients using a home OCT device.6
New monitoring for a new age
The advent of long-acting drugs and slow-release systems that aim to reduce the treatment burden on patients and caregivers calls for concomitant monitoring services that can remotely monitor fluid volumes in patients with active disease and can monitor fellow eyes for conversion to wet AMD. These expected synergies between innovative treatments and diagnostics draw parallels to the early days of the anti-VEGF and SD-OCT era, during which the latter technology paved the way for practical use of the former.
Adequate insurance coverage will be needed to motivate monitoring service providers and treating physicians to implement such new patient management paradigms. The dedicated billing codes for remote OCT recently established in the US lay a good foundation. Recurring billing opportunities with zero capital investment to a practice are expected to drive adoption of this next generation of diagnostic imaging.
References
1. Ho AC, Kleinman DM, Lum FC, et al. Baseline visual acuity at wet amd diagnosis predicts long-term vision outcomes: an analysis of the IRIS registry. Ophthalmic Surg Lasers Imaging Retina. 2020;51:633-639.
2. Chew EY, Clemons TE, Bressler SB, et al; AREDS2-HOME Study Research Group. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121(2):535-544.
3. Ho AC, Heier JS, Holekamp N et al. Real-world performance of a self-operated home monitoring system for early detection of neovascular age-related macular degeneration. J Clin Med. 2021, 10,1355.
4. Nahen K, Benyamini G, Loewenstein A. Evaluation of a self-imaging SD-OCT system for remote monitoring of patients with neovascular age related macular degeneration. Klin Monatsbl Augenheilkd. 2020;237(12):1410-1418.
5. Keenan TDL, Goldstein M, Goldenberg D, Zur D, Shulman S, Loewenstein A. Daily self-imaging with patient-operated home OCT in neovascular age-related macular degeneration. Ophthalmol Sci. 2021;1(2):100034.
6. Heier JS, Holekamp N, Prospective longitudinal study: fluid quantification from daily self-imaging with home OCT in neovascular age-related macular degeneration (NV-AMD). Presented at American Society of Retina Specialists Meeting; San Antonio, TX; 2021.
Newsletter
Keep your retina practice on the forefront—subscribe for expert analysis and emerging trends in retinal disease management.