Novel therapies could stem burden of diabetic eye disease
A better understanding of the pathophysiologic mechanisms of diabetes has resulted in improved control of its local and systemic comorbidities. Further developments are needed, however, considering the growing number of diabetic patients and who are at risk for late-stage diabetic eye disease.
Reviewed by Justus G. Garweg, MD
Dr. GarwegA better understanding of the pathophysiologic mechanisms of diabetes has resulted in improved control of its local and systemic comorbidities. Further developments are needed, however, considering the growing number of diabetic patients and who are at risk for late-stage diabetic eye disease.
“We have been on a good course, but we still have a long way to go,” explained Justus G. Garweg, MD, Berner Augenklinik am Lindenhofspital, Swiss Eye Institute of Bern, Switzerland. “Promising strategies are being evaluated, and there are a number of biologicals and other drugs in the clinical pipeline.
“Future progress, however, will require very close cooperation with our medical colleagues because combined therapeutic strategies at the systemic and local levels will be needed for managing the different underlying systemic comorbidities,” Dr. Garweg added.
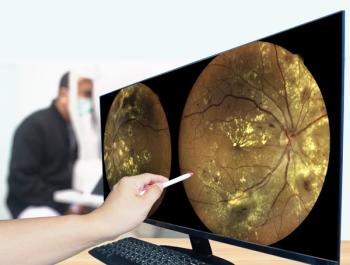
Anti-VEGF injections are considered the standard of care for DME. Results from clinical trials show that on average, patients in real life will gain 7 to 8 letters of visual acuity, with about 50% of patients benefiting with an improvement of 3 or more lines and about 75% having at least a 2-line gain. (Image courtesy of Justus G. Garweg, MD)
Current status
Anti-VEGF injections are considered the standard of care for diabetic macular edema (DME), and results from clinical trials show that on average, patients in real life will gain 7 to 8 letters of visual acuity, with about 50% of patients benefiting with an improvement of 3 or more lines and about 75% having at least a 2-line gain.
Achieving these benefits can be demanding, at least in the first year of treatment where patients seem to need up to 9 injections. Data from follow-up through 4 to 5 years show, however, that the injection requirement decreases thereafter.
Dr. Garweg observed that even patients who seem to be responding insufficiently to initial therapy can benefit from ongoing treatment. He noted that perhaps 40% of patients treated with anti-VEGF injections have persistent DME at 6 months.
With continued treatment, 40% of those patients have chronic persistent DME at 3 years. Within the latter subgroup, about 40% of patients will still have gained at least 10 letters of visual acuity, whereas only 13% will have lost 10 or more letters.
“If you stop treating these patients, they will lose any benefit they achieved,” said Dr. Garweg.
Other targets
Clearly, anti-VEGF treatment is not a general solution for treatment of diabetic eye disease–as up to 30% of patients with diabetic retinopathy have an incomplete response to treatment and in about 40% of patients, proliferative diabetic retinopathy (PDR) is not sufficiently controlled.
The explanation for the limited success achieved with anti-VEGF treatment may be related to uncontrolled inflammation, considering evidence that vitreous levels of inflammatory mediators in eyes of patients with PDR are significantly higher than in controls without diabetes and increase as diabetic retinopathy progresses.
“Control of inflammation may be an insufficiently addressed therapeutic target for diabetic eye disease,” Dr. Garweg said.
He noted that adipocytes may be one source of these cytokines at the systemic level. In that regard, it is noteworthy that gastric bypass or banding, though they do not reduce the number of adipocytes, lead to improvements in blood glucose control and inflammatory markers.
Furthermore, systemic treatment of patients with PDR using the interleukin-1 receptor antagonist anakinra (Kineret, Amgen Inc.) has been shown to improve hyperglycemia and rheumatological activity and to stabilize visual function, albeit without an effect on markers of systemic inflammation.
Similarly, treatment with systemic interleukin-1beta inhibition using canakinumb (Ilaris, Novartis) in patients with PDR was shown to stop progression of retinal neovascularization, stabilize visual acuity and induce a mild reduction in retinal edema together with an improvement in glycemic control measured by HbA1c, again without a change in systemic inflammatory markers.
Role of immune tolerance
Induction of immune tolerance represents another intriguing approach. Dr. Garweg cited research in which islet cells were transplanted in the anterior chamber of the eye.
“These cells showed good survival and were well tolerated under topical corticosteroids,” he said. “During early follow-up, the treated patient had improvement in c-peptide and blood glucose.
“It is hoped that this approach might induce immune tolerance to systemically transplanted islet cells and result in a new endogenous source of insulin production with an improvement in the outcome of diabetes and better control of diabetic retinopathy,” Dr. Garweg added.
Similarly, it was reported in an animal model that interphotoreceptor-binding protein injection into the anterior chamber induced immune tolerance and prevented progression of diabetic retinopathy.
“The treatment did not prevent systemic diabetic organ damage, however,” Dr. Garweg pointed out. “Therefore, the future will depend on intensified interdisciplinary networking to develop combination local and systemic therapeutic strategies.”
Justus G. Garweg, MD
E:
This article is based on a presentation given by Dr. Garweg at the 2017 Retina World Congress. Dr. Garweg is an advisor to AbbVie, Alcon Laboratories, Allergan, Bayer, and Novartis. He also is an investigator in clinical trials of treatment for diabetic retinopathy and age-related macular degeneration, sponsored by Novartis and Bayer. He has declared no conflicts of interest with the presented data.
Newsletter
Keep your retina practice on the forefront—subscribe for expert analysis and emerging trends in retinal disease management.