Resolve patient confusion with AMD awareness, understanding
Age-related macular degeneration (AMD) is the leading cause of significant visual acuity loss in people over the age of 50 in developed countries. Almost 80% of the people diagnosed with AMD will have the non-neovascular (dry) or atrophic subtypes. Here is the clinical information patients need to understand this disease.
By Michelle Dalton, ELS
Age-related macular degeneration (AMD) is the leading cause of significant visual acuity loss in people over the age of 50 in developed countries. Almost 80% of the people diagnosed with AMD will have the non-neovascular (dry) or atrophic subtypes.1, 2
The most advanced form of non-neovascular AMD, known as geographic atrophy (GA), can occur as early as intermediate AMD or in advanced AMD.2 To date, daily intake of antioxidant vitamins is the only commercially available therapy to prevent dry AMD, and even vitamin use is not a universally accepted treatment.3
Most patients will be referred to a retina specialist once a diagnosis of AMD is reached, but they are likely to question their primary eye care professional about the disease before that referral occurs. Both central vision and color perception are affected by AMD, and these tend to develop slowly.
The earliest clinical signs of disease are drusen. One of the earliest symptoms of late AMD is seeing straight lines that appear crooked/blurred. If drusen are small and distinct, there is a reduced risk for progression to advanced AMD. Large, soft, and confluent drusen are more predictive.
Classifying AMD
Although the literature has attempted to establish a clear nomenclature, there remains no universally accepted definition beyond atrophic or neovascular. Classifications range from a general description from the Age-Related Eye Disease study to a Delphi panel description.4, 5
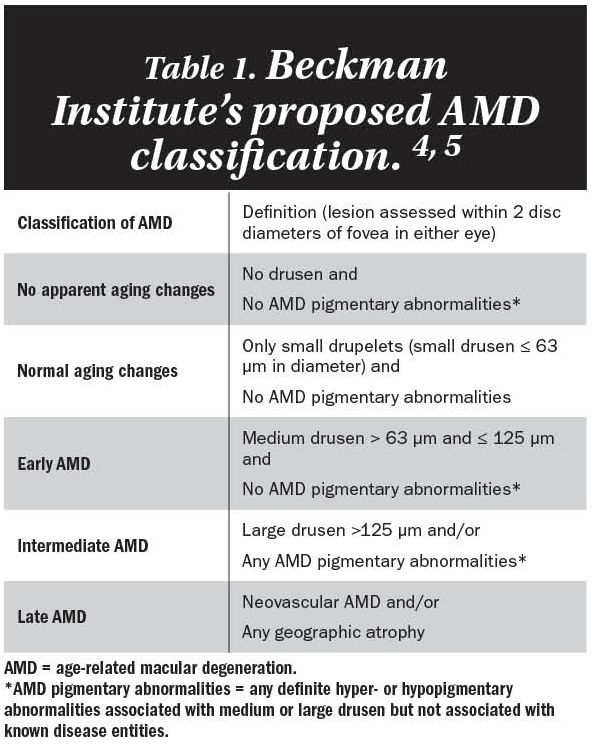
The latter, as part of the Beckman Institute for Macular Research Classification Committee, found the terms “early” and “intermediate” to have multiple definitions, depending on which classification scheme was being employed. (Editor’s Note: In 2016, the Beckman Institute was renamed the Stephen J. Ryan Initiative for Macular Research in honor of its founder.)
Most clinicians will have simplified the approach by qualifying the disease into two categories: early and late. Late disease would include GA and neovascular AMD, and everything else remains “early.” Table 1 describes the Beckman Institute’s proposed AMD classification scheme.
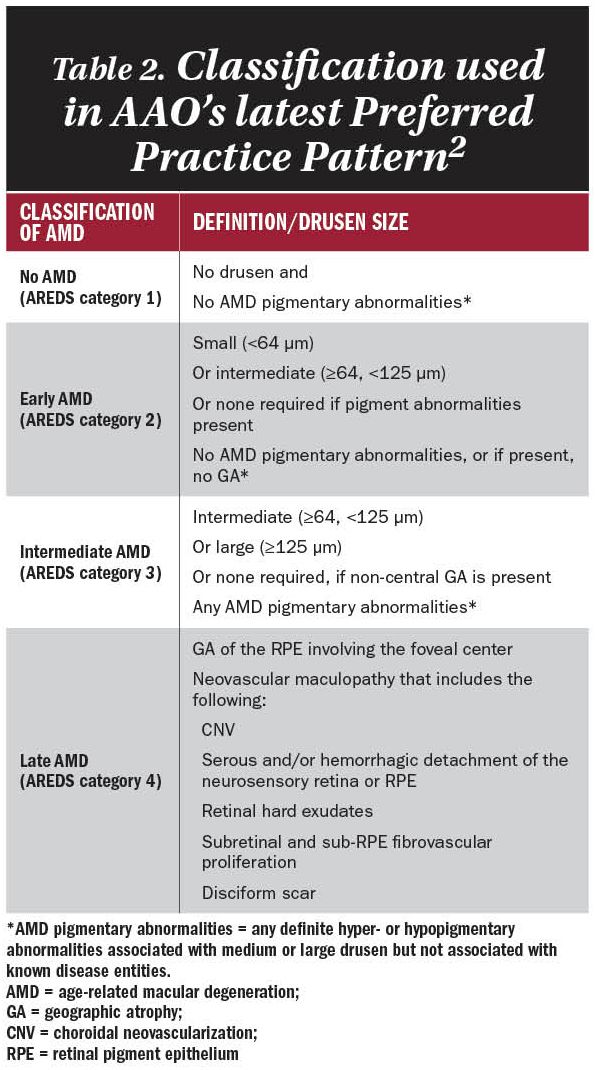
The American Academy of Ophthalmology in its most recent Preferred Practice Pattern (2015) uses the classifications described by the Age-Related Eye Disease Study (AREDS) and the Delphi Panel. See Table 2.
When patients ask about risk
There is a risk calculator available on the National Eye Institute (NEI) website that can help predict the likelihood of a patient developing either GA or neovascular AMD.
According to NEI, the calculator is designed for “individuals between the ages of 55 and 80 years without advanced AMD in either eye, or with advanced AMD in only one eye. For individuals older than 80 years of age, a conservative risk estimate could be obtained by entering 80 years.” (The calculator [http://caseyamdcalc.ohsu.edu] is courtesy of Michael L. Klein, MD, and the Casey Eye Institute/University of Oregon.)
Recently, a set of standardized patient-centered outcome measures in AMD was proposed6 that includes visual functioning and quality of life, number of treatments, treatment complications, and disease control. Standardizing these outcome measures is “crucial in order to direct improvements by those providing treatment, promote dissemination of best practices, and ultimately drive competition around quality.”6
What about dry AMD?
The natural history of dry AMD is progressive, yet many patients with dry AMD remain asymptomatic and unaware of the disease. About 10-15% of the people with dry AMD have rapid deterioration in vision and suffer significant vision loss due to geographic atrophy (GA).7, 8
The American Macular Degeneration Foundation says “nearly everyone over the age of 50 has at least one small drusen,” one of the hallmark signs of dry AMD, and vision loss may still occur.9 The risk factors for developing either form are the same, and people with any two of these factors are recommended to have a complete eye exam.10
References:
1. Kahn HA, Leibowitz HM, Ganley JP, et al. The Framingham Eye Study. I. Outline and major prevalence findings. Am J Epidemiol 1977;106:17-32.
2. American Academy of Ophthalmology Retina Panel. Preferred Practice Pattern: Age-related Macular Degeneration. San Francisco, CA: AAO, 2015.
3. Buschini E, Fea AM, Lavia CA, et al. Recent developments in the management of dry age-related macular degeneration. Clin Ophthalmol 2015;9:563-74.
4. Ferris FL, 3rd, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology 2013;120:844-51.
5. Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119:1417-36.
6. Rodrigues IA, Sprinkhuizen SM, Barthelmes D, et al. Defining a Minimum Set of Standardized Patient-centered Outcome Measures for Macular Degeneration. Am J Ophthalmol 2016;168:1-12.
7. Hageman GS, Gehrs KM, Johnson LV, Anderson DF. Age-related macular degeneration. In: Kolb H, Fernandez EJ, Nelson RW, editors. Webvision: The Organization of the Retina and Visual System [Internet]. Salt Lake City, UT: University of Utah Health Sciences Center, 2008:1573-624.
8. Schmitz-Valckenberg S, Sahel JA, Danis R, et al. Natural History of Geographic Atrophy Progression Secondary to Age-Related Macular Degeneration (Geographic Atrophy Progression Study). Ophthalmology 2016;123:361-8.
9. American Macular Degeneration Foundation. Dry vs Wet Age-Related Macular Degeneration. Available at: https://www.macular.org/dry-vs-wet-macular-degeneration. Accessed June 6, 2016.
10. American Academy of Ophthalmology. Top 5 Risk Factors for AMD. Available at: http://www.aao.org/eye-health/news/top-5-risk-factors-amd. Accessed June 6, 2016.
Newsletter
Keep your retina practice on the forefront—subscribe for expert analysis and emerging trends in retinal disease management.