Study examines silicone rubber balloon scleral buckling for RRDs
Silicone rubber balloon scleral buckling is a novel alternative to the current extraocular procedures for treating simple rhegmatogenous retinal detachments. Investigators have found that this surgery may be a novel alternative to the current extraocular procedures for simple RRD.
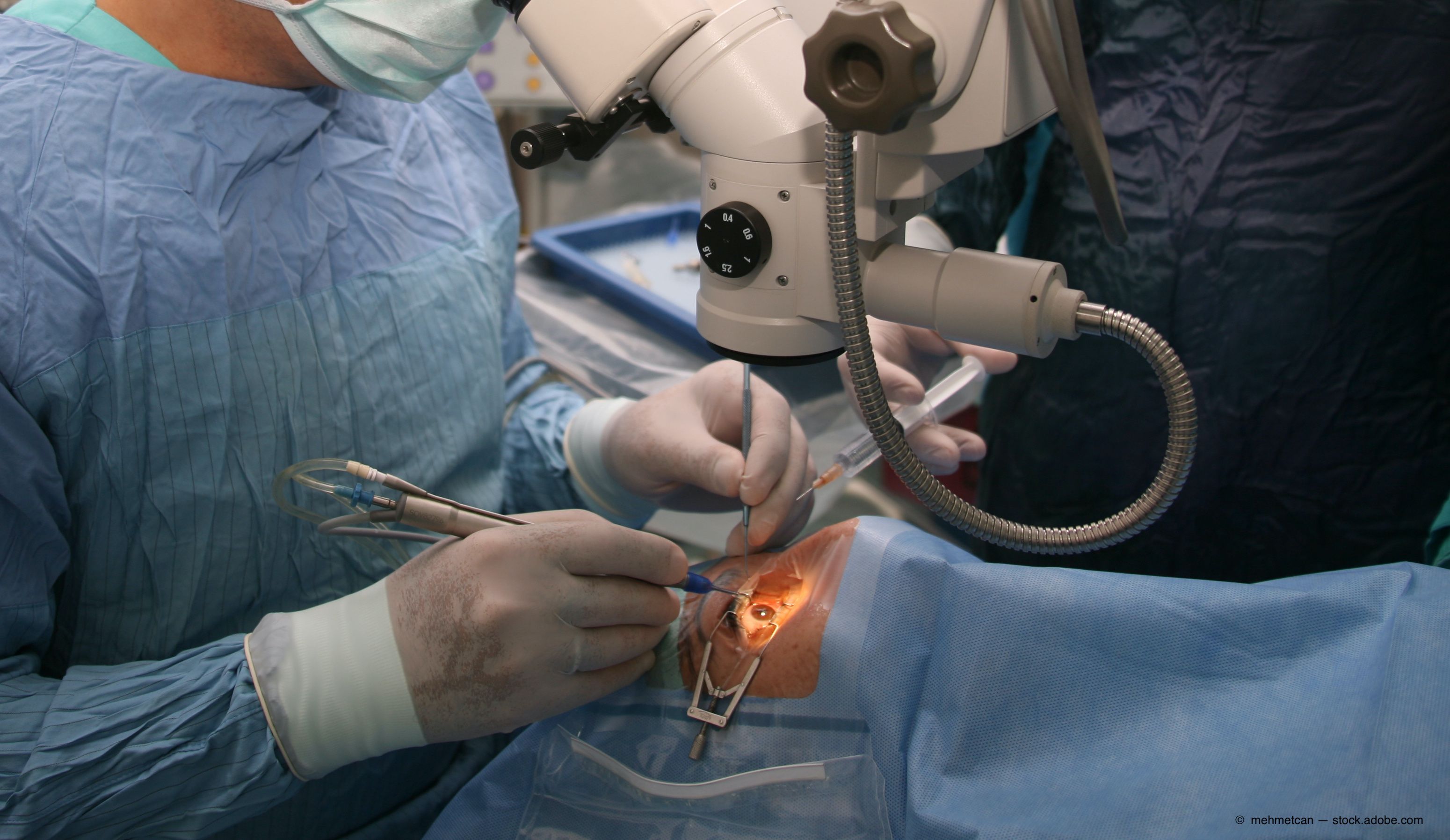
The use of a foldable capsular vitreous body scleral buckle (FCVB) may be a safe and effective alternative method to the currently performed extraocular procedures used to address simple rhegmatogenous retinal detachments (RRDs).
These procedures include scleral buckling, pars plana vitrectomy (PPV), and pneumatic retinopexy. While they are associated with high success rates, all have accompanying disadvantages.
Scleral buckling has been the standard of care for RRDs for decades. It has the advantage of being an extraocular procedure compared with PPV, is not associated with cataract formation, and no posterior vitreous detachment has to be present.
Related: Persistent retinal detachment associated with retinoblastoma
However, investigators pointed out that scleral buckling is associated with use of more anesthesia, the need for higher surgical skills, and changes in refractive error or development of diplopia.
Innovations
One improvement introduced in scleral buckling includes the Lincoff temporary balloon buckle, in which an “inflatable silicone explant that is inserted through the conjunctiva at the ora serrata and that then expands beneath the retinal break to achieve a buckling effect that closes the break and allows subretinal fluid to be absorbed,” according to lead study author Baike Zhang, MD, fromThe People's Liberation Army 988th Hospital in Zhengzhou, China.
Dr. Zhang added that in contrast to conventional scleral buckling, no scleral suturing is required — thus eliminating the risk of scleral perforation and less surgical trauma and resulting in a shorter operating time.
However, this balloon is not commercially available and a long pipe is left for an extended time outside the eye, which is uncomfortable and inconvenient.
As a step forward in scleral buckling innovations, Zhang said he and his colleagues began using the FCVB as the material for scleral buckling to treat RRD, with the hope of making the procedure “easier, more convenient, and painless for the patients compared with the conventional scleral buckling surgery.”
Related: Improving visual outcomes after 360° relaxing retinectomy
The FCVB used in this study was the AV-10P (Vesber, Guangzhou, China), made of silicone rubber and consisting of a thin vitreous cavity–shaped capsule with a tube–valve system. The BSS can be injected into the capsule through the tube–valve system, which exerts pressure on the sclera.
Another advantage is that this procedure has a shorter tube–valve system that can be placed under the conjunctiva and reduces the risk of orbital infection.
Pilot study
Dr. Zhang and colleagues conducted a clinical trial that included 5 patients (age range of 26 to 62 years) with simple RRDs who were treated with the FCVB.
The patients were followed for a minimum of 12 weeks, during which time the treatment was evaluated for efficacy and safety based on the presence of infection, pain, diplopia, elevated intraocular pressure, and other serious postoperative complications after surgery.
The investigators reported, “The simple RRD in all five patients was reattached successfully before being evaluating by B-ultrasound and fundus photography postoperatively. The visual acuity was enhanced in 2 patients in whom the macula was involved. One patient had temporary diplopia and eye movement limitation after surgery. No other complications were recorded.” They reported their results in Retina.1
No adverse events occurred. Diplopia developed in 1 patient, which can resolve with the removal of the scleral buckle.
Related: Looking to the future in retina innovations
Based on these finding, the FCVB scleral buckling was considered efficacious and safe for treating simple RRD.
“The results indicate that this surgery may be a novel alternative to the current extraocular procedures for simple RRD,” the investigators concluded.
The FCVB has been approved by the China Food and Drug Administration, obtained CE certification for distribution and use in the European Economic Area, and has been clinically used in these countries.
Baike Zhang, MD
E: 13949005500@163.com
The authors have no financial interest in this subject matter.
--
Reference
1. Zhang B, Li C, Jia Y, et al. A pilot clinical study of treatingrhegmatogenous retinal detachmentby silicone rubber balloon scleral buckling. Retina 2020;40:1918-28. doi: 10.1097/IAE.0000000000002685
Newsletter
Keep your retina practice on the forefront—subscribe for expert analysis and emerging trends in retinal disease management.