Telemedicine making ROP screening and monitoring a viable reality
By Michelle Dalton, ELS;Reviewed by Antonio Capone, Jr., MD
With tele-healthcare use on the rise despite its barriers, it is surprising that its penetration in retinopathy of prematurity (ROP) screening is not higher than 21%, according to Antonio Capone, Jr., MD.
A survey of medical directors at 393 level III neonatal intensive care units found 44% of the directors did not think there were enough ophthalmologists to screen and/or treat ROP in their local area.1
ROP surveillance is a workflow process, with “an established disease management algorithm,” said Dr. Capone (see Figure 1), adding numerous studies have demonstrated the validity of the telemedicine paradigm for ROP surveillance.
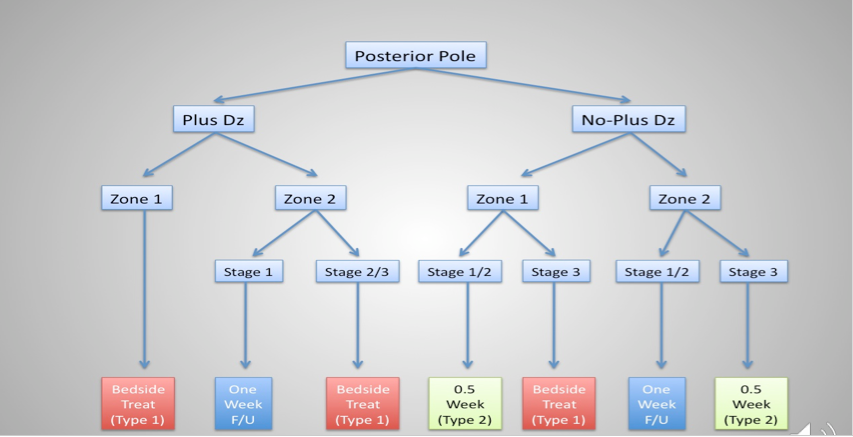
Figure 1. Established disease management algorithm for retinopathy of maturity. (Image courtesy of Antonio Capone, Jr., MD)
Dr. Capone is clinical professor of biomedical sciences, Oakland University William Beaumont School of Medicine, Rochester Hills, MI, and is in private practice at Associated Retinal Consultants, Royal Oak, MI.
Digital fundus imaging, performed weekly, “[fortifies] the safety net for eyes where aggressive disease can be missed,” after initial screening, Dr. Capone said.
“Digital imaging is more cost effective and significantly more time efficient for physicians,” he added. “A further advantage is that digital data are available for objective review, dissemination, and second-opinion consultation.”
For infants followed with digital imaging, ophthalmoscopy is performed within 72 hours of the final imaging session as a segue to outpatient surveillance. Finding Zone 1 and plus disease is easier with digital imaging, and are “more cost effective and significantly more time-efficient,” he said. Both are key advantages in view of the manpower challenges of ROP and the high screen-to-treat ratio. Software programs can further delineate findings.
Dr. Capone listed both didactic (www.focusrop.com/home/education) and case-based (aao.org/pediatric-center-detail/retinopathy-of-prematurity-case-based-training) online resources as useful education tools for clinicians.
Digital advantage
Digital screening offers the additional benefit of lending itself to informatics analyses, a field led by Michael Chang, MD; Peter Campbell, MD; and colleagues at the University of Oregon.
“The greatest potential of digital imaging is not how we use it today, but in what the future holds in bringing the power of machine learning to bear on the problem of ROP diagnostics,” Dr. Capone said, adding that deep learning is not a panacea.
The topic is increasingly being mentioned in retina publications, he said, primarily in imaging and diabetic retinopathy articles.
“In ROP, changes to just a few pixels in a retinal image may cause the algorithm to miss disease readily apparent to human graders, or clinician-mimicking algorithms,” he said.
Physicians will not be replaced, Dr. Capone pointed out, but they should embrace digital imaging to enable better patient care.
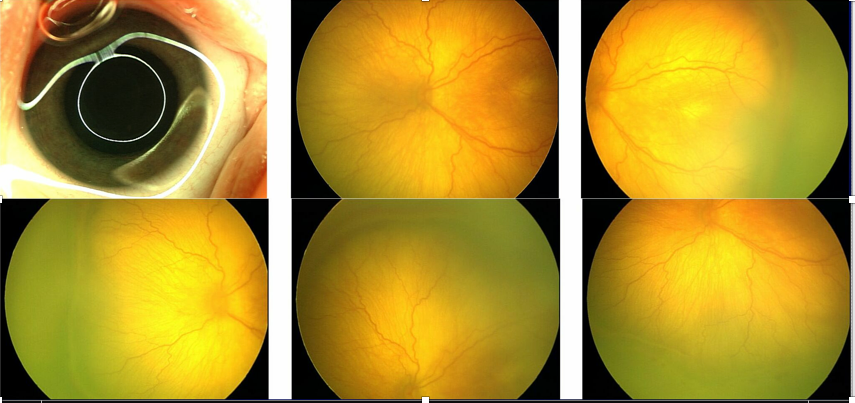
Figure 2. Digital fundus imaging require multiple images to maximally capture fundus details. (Image courtesy of Antonio Capone, Jr., MD)
A 2014 report from the American Academy of Pediatrics (doi: 10.1542/peds.2014-0978) embraced telemedicine for ROP. At Dr. Capone’s facility in Michigan, digital fundus imaging options are limited to devices that require multiple images “to maximally capture fundus details” (see Figure 2).
Pan-fundus, digital-imaging technologies are lacking, Dr. Capone said. Ironically, while the first-generation devices made telemedicine for ROP a reality, the cost was more than one could imagine. That may have resulted in limiting the widespread adoption of telemedicine technology for ROP surveillance.
Future of digital imaging
Smartphone devices can capture high-quality images (either with or without hardware attachments), but are limited with regard to field of view. Dedicated digital fundus cameras provide the highest quality images, and “are finally evolving” in the pediatric field.
Cost remains an issue, and will continue to limit the use and market penetration, Dr. Capone said. Ultra widefield imaging is not yet available. But any discussion on imaging would be “incomplete” without the mention of evolving optical coherence tomography (OCT) technologies, he said.
“OCT promises to bring depth to the current two dimensional construct,” Dr. Capone said, citing the work being done on 3D techniques for analyzing vascular findings by Cynthia Toth, MD, and colleagues at Duke University.
“There are early reports of ROP eyes imaged using both commercially available, and prototype OCT-angiography technology designed specifically for ROP” that are showing promise, he said.
Digital health technology “can reduce healthcare costs and improve access and outcomes for infants at risk for ROP,” he said. “More than 15 years ago, I spoke about the future of telemedicine. That future is now.”
Reference:
1. Vartanian RJ, Besirli CG, Barks JD, et al. Trends in the Screening and Treatment of Retinopathy of Prematurity. Pediatrics.2017;139(1):e20161978
Antonio Capone, Jr., MD
p. 248-288-2280
This article is adapted from a presentation the Dr. Capone delivered at the Retina Subspecialty Day, prior to the 2017 American Academy of Ophthalmology meeting.
Dr. Capone is an equity owner in Broadspot, FocusROP, Phoenix Technology Group, and Retinal Solutions. He also holds patents and receives royalties from Retina Solutions. He also received grants, patents/royalties, and is an advisor to other ophthalmic companies unrelated to the subject matter.
Newsletter
Keep your retina practice on the forefront—subscribe for expert analysis and emerging trends in retinal disease management.