UC Irvine researchers discover a nanobody which may lead to treatment for retinitis pigmentosa
According to the university, a special antibody that is derived from llamas, called a nanobody, can stop the misfolding and the activation of Rhodopsin, a molecule whose mutations can lead to blindness.
(Image Credit: ©ashtproductions - Adobe.Stock.com)

A team of scientists from the University of California Irvine (UCI) believe they have discovered a special antibody which may ultimately lead to a treatment for retinitis pigmentosa.
The study1 was published in Nature Communications. Authors of the study were Arum Wu, PhD, David Salom, PhD, John D. Hong, Aleksander Tworak, PhD, Philip D. Kiser, PharmD, PhD, and Krzysztof Palczewski, PhD, in the Department of Ophthalmology, Gavin Herbert Eye Institute, at the University of California, Irvine.
According to a UCI news release, there currently is no known cure for RP, and the development of new treatments for this condition relies on cell and gene therapies.
The UCI researchers focused their efforts on a specific molecule which they believe will provide a treatment for Rhodopsin-associated autosomal dominant RP (adRP). The molecule, Rhodopsin is a key light-sensing molecule in the human retina. It is found in rod photoreceptor cells, and mutations in the Rhodopsin gene are a primary cause of adRP.1
“More than 150 mutations in rhodopsin can cause Retinitis Pigmentosa, making it challenging to develop targeted gene therapies,” Krzysztof Palczewski, PhD, Donald Bren Professor, UCI School of Medicine, said in the UCI news release. “However due to the high prevalence of RP there has been significant investment in research and development efforts to find novel treatments.”
This image depicts the crystal structure of two nanobodies binding to a rhodopsin dimer. The rhodopsin molecules are shown in green and blue, with 11-cis-retinal displayed in red. The figure emphasizes the significant interactions between the nanobodies (represented in a semi-transparent surface cartoon) and the extracellular surface of rhodopsin, including its N-terminal glycans highlighted in orange. (Image courtesy of University of California Irvine)
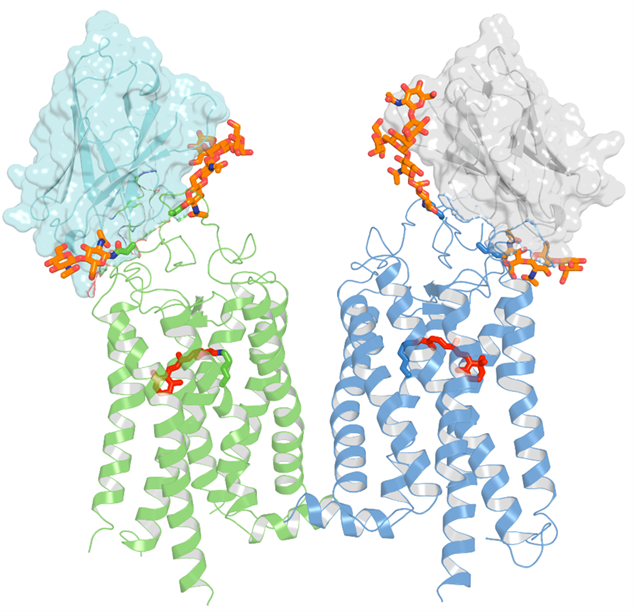
Researchers have studied Rhodopsin for more than a century, according to the release, and key details of its mechanism for converting light into a cellular signal have been a challenge to address in experiments.1 For this study, researchers used a special type of llama-derived antibody, known as a nanobody, that can halt the process of Rhodopsin photoactivation, allowing it to be investigated at high resolution.1
David Salom, a researcher and project scientist, UCI School of Medicine, said in the UCI news release, the team has developed nanobodies that work through a novel mechanism of action.
“These nanobodies have high specificity and can recognize the target rhodopsin extracellularly,“ Salom said in the UCI news release. “This enables us to lock this GPCR in a non-signaling state.”
Moreover, according to the UCI news release, the scientists discovered that these nanobodies target an unexpected site on the Rhodopsin molecule, near the location where retinaldehyde binds. The team also discovered that the stabilizing effect of these nanobodies ultimately could also be applied to Rhodopsin mutants that are associated with retinal disease, suggesting their use as therapeutics.1
“In the future, we hope to involve the in vitro evolution of these initial set of nanobodies,” Arum Wu, PhD, researcher and project scientist, UCI School of Medicine, said in the news release. “We will also evaluate the safety and effectiveness of a future nanobody gene therapy for RP.”
Researchers hope to improve nanobodies' ability to recognize Rhodopsin from other species including mice, for which several pre-clinical models of adRP are available, the release concluded, adding that the investigators also have plans to use these nanobodies to address a long-term goal in the field of structurally resolving the key intermediate states of Rho, from the inactive state to the fully ligand-activated state.1
Reference:
Wu, A., Salom, D., Hong, J.D. et al. Structural basis for the allosteric modulation of rhodopsin by nanobody binding to its extracellular domain. Nat Commun 14, 5209 (2023). https://doi.org/10.1038/s41467-023-40911-9