UCI study cites base editing as a durable one-time treatment for inherited retinal degeneration
According to researchers at the University of California, Irvine, base editing may provide long-lasting retinal protection and prevent vision deterioration in patients with inherited retinal degeneration, specifically in Leber congenital amaurosis patients.
Researchers show that in vivo correction of an Rpe65 mutation by adenine base editor (ABE) prolongs the survival of cones in LCA mouse model. (Image provided by University of California, Irvine)
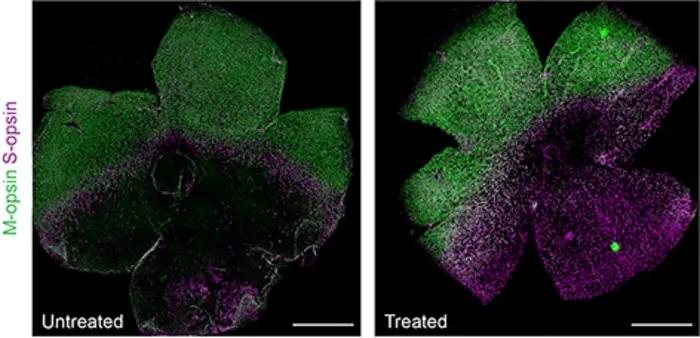
A University of California, Irvine-led study indicates base editing may provide long-lasting retinal protection and prevent vision deterioration in patients with inherited retinal degeneration, specifically in Leber congenital amaurosis (LCA) patients.
“Our findings demonstrate the tremendous potential of base editing,” senior author Krzsztof Palczewski, PhD, Donald Bren Professor of Ophthalmology at the UCI School of Medicine, said in a news release. “If scientists align behind this approach, there is a chance that in 10 years, all inherited retinal diseases could be treatable.”
According to the university, the study, “In vivo base editing rescues cone photoreceptors in a mouse model of early-onset inherited retinal degeneration,” was published in Nature Communications.
In the release, the university noted that LCA is the most common cause of inherited retinal degeneration in children. LCA patients with RPE65 mutations show accelerated cone photoreceptor dysfunction and death, resulting in early visual impairment. It is therefore crucial to develop a robust therapy that not only compensates for lost RPE65 function, which impair the visual cycle and contribute to retinal degeneration, but also protects photoreceptors from further degeneration.
“In this study, we found that the base editing treatment can rescue the function and survival of cone photoreceptors on a long-term basis in the rd12 mice, which exhibit rapid cone photoreceptor degeneration,” Palczewski said in the news release. “Protecting photoreceptors is key to preventing further deterioration of vision in LCA patients, which means this discovery could lay the foundation for establishing the therapeutic potential of base editing as a one-time, durable treatment.”
A common clinical manifestation of LCA2 is early cone dysfunction and degeneration in the fovea—a small, central pit composed of closely packed cones in the eye—and the parafovea, which surrounds the fovea. The fovea and parafovea comprise the essential structure of the retina responsible for the highest visual acuity necessary for reading, driving and performing many daily activities. Because the cone photoreceptors in the fovea are crucial for central vision and essential visual acuity, prevention of cone degeneration is a primary goal for therapeutic intervention in LCA2 patients.
In recent years, gene therapy has taken major strides forward with successful clinical trials of RPE65 augmentation therapy, which ultimately led to approval by the U.S. Food and Drug Administration (FDA). It has created new hope for LCA patients by improving their visual acuity. Yet, long-term outcomes regarding the progression of retinal degeneration are still controversial.
The university noted that during this same time, base editing has rapidly emerged as a potential approach to treat genetic disorders, with promising outcomes in different preclinical models. The base editing approach has great promise especially for targeting genetic eye disorders, given the unique therapeutic advantages of the eye, and the prior demonstration of successful genetic rescue in a mouse model.
“It is our belief, that with some further development, base editing will provide a new paradigm for the treatment of numerous inherited ocular diseases caused by different modes of inheritance,” Palczewski said in the news release.
According to the university, additional preclinical testing in non-human primates with foveas, will be necessary before this approach can be tested in patients. Furthermore, alternative delivery methods should be investigated to cover a broader area of the RPE tissue in patients and to circumvent constitutive base editor expression, which could induce unwanted DNA/RNA editing and immune reactions in the long term.
The university noted that the research was supported by the National Institutes of Health, Research to Prevent Blindness, the National Science Centre and the International Centre for Translational Eye Research.