Video system has ‘transformative potential’ for blind patients
By Michelle Dalton, ELS;Reviewed by Robert Devenyi, MD
A digital device worn like a pair of spectacles may have the potential to be “transformative” as a low-vision aid, allowing the legally blind to actually see, be mobile, and independently engage in all activities of daily living.
The eSight system (eSight Corp., Toronto, Canada) is a set of electronic glasses, which look similar to the 3D glasses that video gamers use, has a controller so users can magnify an image to great extents. Time magazine named the system one of the 25 best inventions of 2017.
“Like other devices, it has an autofocus, but what really sets it apart from other devices is it has bioptic tilt,” said Robert G. Devenyi, MD, FACS, FRCSC. Dr. Devenyi is a vitreoretinal surgeon; professor of ophthalmology and vision sciences, University of Toronto.
Bioptic tilt allows the patient to tip the device up a small amount above their central vision, but allows retention of the patient’s native peripheral vision to navigate. When patients do tip the device upwards, the camera system remains focused centrally so the patient can walk around, use his or her peripheral vision, but quickly glance up to see in detail what is in front of them. The company notes the bioptic tilt helps ensure a patient’s balance and prevents nausea that might occur with other immersive technologies.
“That is what makes this so incredibly life changing for patients,” Dr. Devenyi added.
Technology behind the system
According to the company, the eSight system is a high-speed, high-definition camera. Its algorithms enhance the video feed and display it on two OLED screens in front of the user’s eyes. Full color video images are seen clearly by the user with unprecedented visual clarity and virtually no lag.
The current generation (eSight 3) boasts a 21.5-m pixel camera, with a 0.9-20x wide zoom range. Field of view is 30º, and the system acuity (Snellen/ETDRS) is 20/12. The device can connect directly to a computer or television via HDMI, WiFi, or Bluetooth technologies.
The eSight 3 is expected to cost just under $10,000, and is a Class 1 Medical device registered in the United States and European Union. It also has been validated by Health Canada, and is available in North America, Europe, Australia, New Zealand, and India. Dr. Devenyi said the company is expanding the number of countries where the device will be marketed.
What the studies report
Clinical studies were completed on the eSight 2. The eSight Quality of Life and Efficacy Study (eQUEST) was a prospective, cohort study intended to demonstrate the functional vision and quality of life improvement provided by eSight Eyewear to persons with significant visual impairment resulting from various eye conditions.
The study evaluated the efficacy of the eSight device for various activities of daily living (ADLs), and was conducted at 6 sites throughout Canada and the United States. At baseline, patients’ best corrected vision had to be between 20/60 and 20/400 on the ETDRS charts.
Patients had to have central or scattered scotomata or geographic atrophy, and a visual field of at least 20º (bilaterally or monocularly). Exclusion criteria included fluctuating vision, surgical procedures, or anti-vascular endothelial growth factor injections within the previous 6 months before enrollment, among others.
The primary outcome was scores on the Veterans Administration Low Vision Visual Function questionnaire that asks 48 questions (VA LV VFQ-48), Dr. Devenyi said. All 51 enrolled subjects filled out the questionnaire at baseline.
They were given a demonstration on how to use the device and were provided customized devices (customized for lens prescriptions) to wear for 3 months, and then re-assessed. Secondary outcomes included visual function, reading speed, facial recognition, and ADLs.
“There were no specific diagnoses that were excluded or included,” Dr. Devenyi outlined. “Diagnoses at baseline included Stargardt macular dystrophy, Leber congenital amaurosis, atrophic age-related macular degeneration, optic atrophy, retinitis pigmentosa, and rod-cone dystrophy, among others.
“It was really more a function of how much central vision they had,” he added. “There were no serious side effects noted.”
Minor adverse events included 1 report each of nausea and mild neck pain.
‘Interesting results’
Dr. Devenyi called the results “interesting,” adding the acuity measurements “were real as we would expect based on the magnification of the device,” with some patients gaining up to 7 lines of improvement (p < 0.001). (See Figure 1.) Contrast sensitivity was dramatically improved (p < 0.001), with a 12-letter immediate gain.
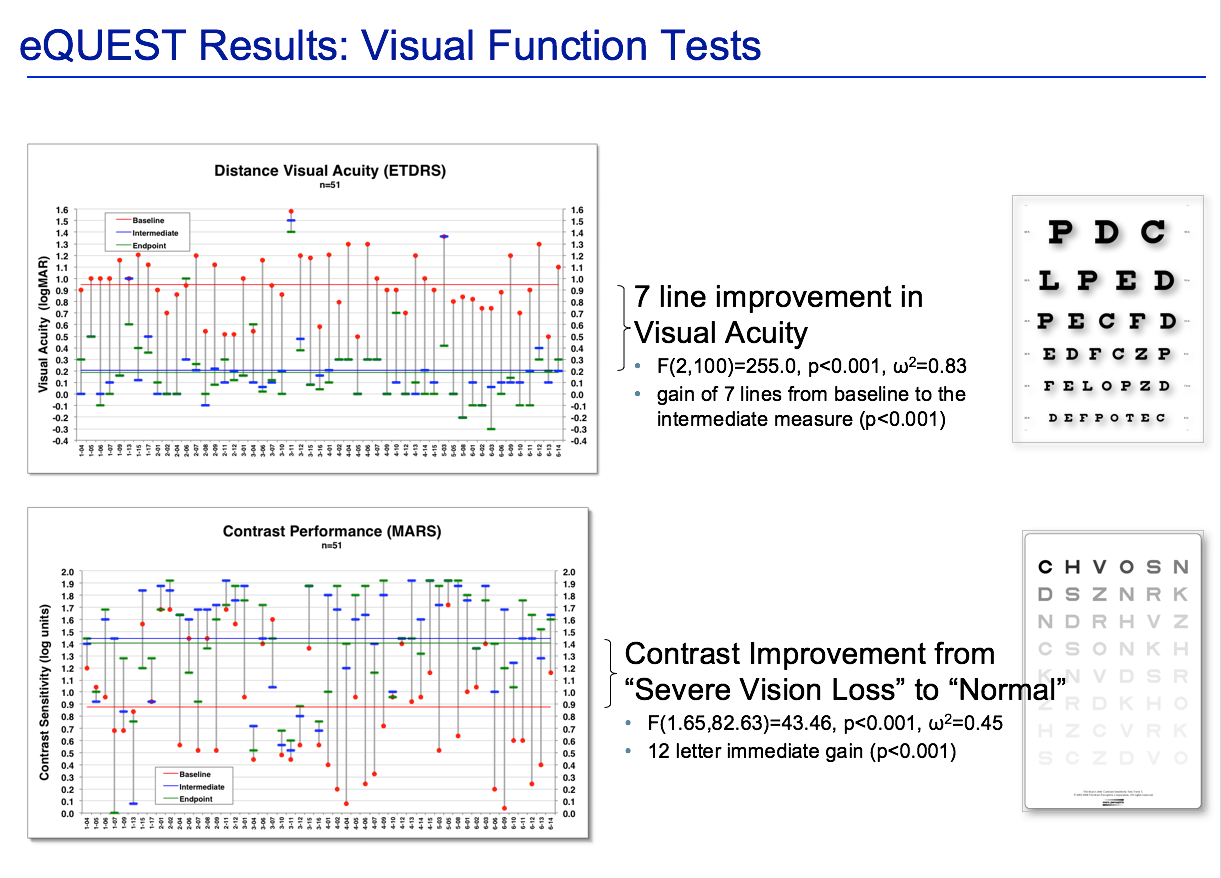
Figure 1. Results from the eQuest study on the low-vision aid eSight 2. The device is clinically validated by many North American eye institutes and health researchers, including the University Health Network, in a study that will be released in a few months. (Image courtesy of Robert G. Devenyi, MD)
All measured factors “were very statistically significant,” he added. Reading was improved by a 6-point font size, with an immediate 5-line gain (p < 0.001) and an additional line gain after 3 months (p = 0.03). There was significant improvement in various ADLs (p = 0.005).
On the VA LV VFQ-48, 80% of the test population showed improved overall total scores (p < 0.001), with only mobility not reaching statistical significance (meaning the device did not impact mobility).
“This is the first device that allows patients to have dramatic improvements in their visual acuity when we have no other therapeutic options for them, and one that does not interfere with their mobility,” Dr. Devenyi said.
The enrolled patients did complain about the size and comfort of the device, but Dr. Devenyi said that issue has been addressed with the eSight 3.
Robert G Devenyi, MD
e. robert.devenyi@uhn.ca
This article was adapted from a presentation Dr. Devenyi delivered at Retina Subspecialty Day, held prior to the 2017 American Academy of Ophthalmology meeting. Dr. Devenyi is a consultant and advisor to the eSight Corp.
Newsletter
Keep your retina practice on the forefront—subscribe for expert analysis and emerging trends in retinal disease management.