
Previously, Teva was established as the European commercialisation partner of FYB201, Formycon's biosimilar to ranibizumab (Lucentis).

Previously, Teva was established as the European commercialisation partner of FYB201, Formycon's biosimilar to ranibizumab (Lucentis).

The objective of this trial was to evaluate safety and tolerability and identify dose level for further evaluation.

Whitecap is currently developing 2 therapies for potential use in glaucoma and geographic atrophy.
OCU400 demonstrated meaningful improvement of 2-line gain (10 letters on ETDRS chart) in low-luminance visual acuity (LLVA) in treated eyes when compared to untreated fellow eyes.

City Therapeutics will develop a novel RNAi clinical candidate toward a specific disease target for intravitreal administration.


This phase 2b study (ASPIRE) is currently underway.


The application was refiled following a December 20, 2024, meeting between the US Food and Drug Administration (FDA) and Astellas.

Several pharmaceutical companies have announced new appointments of executives and board members as they prepare for growth in 2025.

The financing will go toward its ongoing phase 2b/3 and planned pivotal phase 3 clinical trial for AVD-104.

ViGeneron also received approval for dose escalation in the ongoing phase 1b clinical trial.

In October, Oxular began a phase 2 trial for its therapeutic candidate OXU-001, for treatment of diabetic macular edema

The primary safety endpoint was carried out through the percentage of patients with shift from normal (at baseline) to abnormal in any electrocardiogram (ECG).

The collaboration between KIST and Huons BioPharma will span 14 months.

Most recently, Jacques served as Vice President of Healthcare Strategy, Partnerships & M&A at Microsoft.
The Danish Medicines Agency requested the EMA's Pharmacovigilance Risk Assessment Committee review reports on the vision-threatening condition

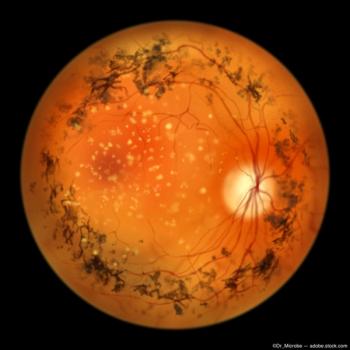
LHON, a genetic disease that affects the retinal ganglion cells, results in severe bilateral sequential vision loss.

The potential gene therapy candidate is being evaluated for geographic atrophy.

The rationale was that commercial mydriatics administered during retinopathy of prematurity (ROP) screening have been associated with cardiorespiratory and gastrointestinal adverse events.

The trial is evaluating GAL-101 eye drops in patients with geographic atrophy, an advanced form of dry AMD.

In this study, researchers examine immune mechanisms in ocular diseases like uveitis, AMD, DR, and GO, highlighting microglial roles, targeted therapies, and promising advances in immunotherapy.

A look at the biggest news and advancements in ophthalmology in 2024.

This year was brimming with advancements in optometry.

The conference will be held January 13-16, 2025 in San Francisco, CA.

A single lot (Lot 10101) of Systane Lubricant Eye Drops Ultra PF, Single Vials On-the-Go, 25 count, is being voluntarily recalled by Alcon Laboratories due to the detection of fungal material in a consumer-reported vial.

Trending topics and milestone stories from 2024 on Ophthalmology Times Europe

A Helsinki University Hospital study found diabetes, glycemic control, or insulin therapy did not significantly affect anatomical or functional outcomes after epiretinal membrane surgery.

Let’s look back on some of the top stories on Modern Retina from 2024 as we gear up for an incredible 2025.