The study will include 100 patients with the aim of determining effective and ineffective standards of eye injections for AMD to better personalize future medical evaluations.

The study will include 100 patients with the aim of determining effective and ineffective standards of eye injections for AMD to better personalize future medical evaluations.

The certification will allow 20/20 Onsite to make clinical trials more accessible to patients nationwide and expand site options for sponsors while maintaining performance and data collection standards, including BCVA and DR testing.
As a nonsteroid treatment, RG6179 could open new methods for controlling inflammation with fewer adverse effects.
In-person and virtual trainings, coupled with the support of local product representatives, are the best ways to ensure retina specialists are set up for success.

This phase will evaluate UNITY’s UBX1325 (foselutoclax) in a head-to-head comparison with aflibercept for the treatment of diabetic macular edema (DME).

How AI tools can drive early detection of diabetic eye conditions.

Modern Retina is looking back at the first half of 2023 as we explore the role of AI in the retinal field in anticipation of the changes to come.

Real-world patients often fail to achieve and retain the visual acuity results observed in clinical trials for anti–VEGF-A therapy. Broader therapeutic inhibition of additional angiogenic factors VEGF-C and VEGF-D could change that.
Clinical benefit of complement inhibition has been demonstrated in clinical trials, but there are possible consequences to consider.

New treatment options enhance patient visual outcomes and reduce treatment burdens.
Antimicrobial resistance is resistance to multiple classes of antibiotics, which makes it extremely challenging to treat infections caused by such organisms.

Artificial intelligence (AI) is going to be highly instrumental in patient care in all medical specialties and will be highly relevant in eye care.

EyeBio has begun the dosing of the first participants in its Phase 1b/2 AMARONE clinical trial for the treatment of diabetic macular edema (DME) and neovascular age-related macular degeneration (nAMD).
Jorge L. Alió, MD, PhD, FEBOphth, shared his pearls situations in which a patient presents with a cataract and corneal opacity at the European Society of Ophthalmology Conference, Prague.

The open source article reports on the anti-inflammatory compound and its potential to treat retinal diseases.


This approval will allow Belite Bio to initiate the phase 3 clinical trial in South Korea, also known as the DRAGON study.


Paracentral acute middle maculopathy can develop after excessive intake of caffeine and alcohol according to Obadah Moushmoush, MD.
Omnigen® may be an alternative treatment option for patients with a persistent corneal epithelial defect. The product provides a convenient and effective treatment that can be administered in an outpatient setting.
This two-in-one option, PreserVision AREDS 2 Formula eye vitamins plus CoQ10 combines the exact nutrient formula recommended by the National Eye Institute (NEI) to help reduce the risk of moderate to advanced Age-related Macular Degeneration (AMD) progression.

The 12-month data demonstrated maintenance of controlled wet AMD subjects comparable to aflibercept injections every eight weeks with a single administration of OTX-TKI.

Investigators have enrolled the 112 patients in the KALAHARI phase 2, part B clinical trial for diabetic macular edema (DME). This is above the originally planned total of 108 patients.

The company’s pipeline includes treatments for XLRP, AMD, and cone-rod dystrophy (CRD).
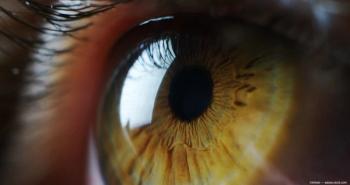
Senescent cells accumulate in areas of disease activity in DME and wet age-related macular degeneration and release mediators that drive the pathology. UBX1325 seems to prevent that disease activity.
Patients who are non-responders to anti-vascular endothelial growth factor (VEGF) therapy may benefit from intravitreal dexamethasone.
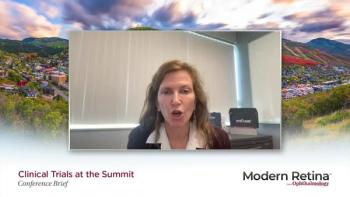
Caroline Baumal, MD shares an update on visual function data from DERBY and OAKS Studies based on her presentation at the 2023 Clinical Trials at the Summit annual meeting, held in Park City, Utah.
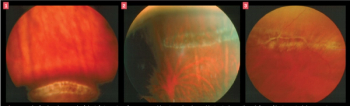
Prompt evaluation and adherence to guidelines for treatment recommendations for lattice retinal degeneration are essential to the preservation of vision and sight.

RAFARM and BioNanoSim have entered into an agreement to create a new Opthalmic company: BNS Opthalmics (BNSO).

OTX-TKI is the Ocular Therapeutix’s axitinib intravitreal implant that is being developed for the treatment of diabetic retinopathy, wet AMD and other retinal diseases.