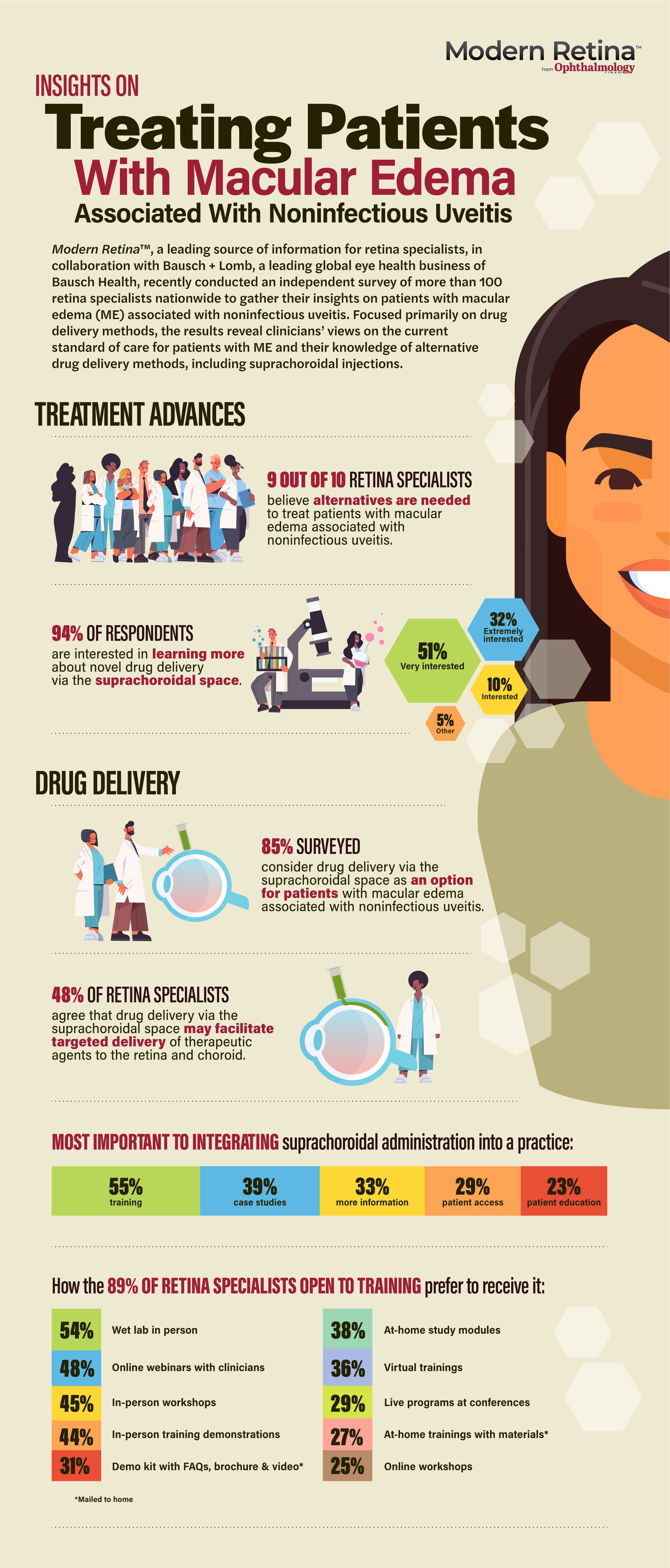
Survey: Retina specialists see need for alternative treatments for macular edema associated with noninfectious uveitis
Survey respondents point to a perceived gap in treatments for uveitic macular edema, document interest in suprachoroidal drug delivery.
A recent independent survey of retina specialists provides insights on their perspectives regarding the management of macular edema associated with noninfectious uveitis. The survey was conducted by Modern Retina™ in collaboration with Bausch + Lomb.
The survey was prompted by the availability of triamcinolone acetonide injectable suspension (Xipere) for suprachoroidal use as a new modality for treating uveitic macular edema. The survey polled over 100 retina specialists across the United States, and the questions were designed primarily to assess clinicians’ views on the current standard of care for treating macular edema associated with noninfectious uveitis and retina specialists’ knowledge about alternative drug delivery methods, including suprachoroidal injections.
The results showed that the respondents overwhelmingly see a need for alternative options to treat the condition that represents the most common sight-threatening complication of noninfectious uveitis. In addition, the survey demonstrated the clinicians had strong interest in learning about drug delivery via the suprachoroidal space.
Digging into the data
Overall, 90% of the retina specialists who participated in the survey agreed that there is a need for alternative treatments for patients with macular edema associated with noninfectious uveitis, and 94% of the respondents were interested in finding out more about drug delivery via the suprachoroidal space. Approximately one-third of the retinal specialists indicated they were “extremely interested” in learning more about suprachoroidal drug delivery, and 51% said they were “very interested”.
Responses to questions focusing specifically on the suprachoroidal route for drug delivery showed that 85% of the surveyed retina specialists consider it an option for treating macular edema associated with noninfectious uveitis. Almost half of the respondents (48%) agreed that this novel method of drug administration may facilitate targeted delivery of therapeutic agents to the retina and choroid.
The procedure for administering a suprachoroidal injection is distinctly different than the intravitreal injection procedure. Perhaps it is not surprising, therefore, 55% of respondents cited the need for training as an important factor for the integration of suprachoroidal administration into clinical care. Case studies and more information were identified as important factors for supporting adoption of the novel drug delivery approach by 39% and 33% of survey respondents, respectively. Patient access was noted to be an important factor by 29% of the retina specialists and 23% identified a need for patient education.
Eighty-nine percent of the retina specialists who participated in the survey were open to receiving training on the suprachoroidal injection technique. The survey results showed wide variation in their preferences for receiving the training. Although the retina specialists’ responses indicated significant interest in several different educational formats, they overall favored in-person programs.
Of the retina specialists who indicated interest in receiving training on suprachoroidal administration, the largest proportion (54%) preferred to receive it via in-person wet lab sessions, 45% preferred attending in-person workshops, 44% preferred in-person training demonstrations, and 29% favored live programs at conferences.
Nearly half of the respondents open to training expressed interest in online webinars with clinicians, 36% said they preferred virtual trainings, and 25% favored online workshops. Sizeable proportions of the retina specialists who were open to receiving training on suprachoroidal injection indicated preferences for at-home (nonweb-based) formats that could consist of using at-home study modules (38%); a demo kit providing answers to frequently asked questions, a brochure, and video (31%); or at-home training using materials received through the mail (27%).
Access to training
Triamcinolone acetonide injectable suspension for suprachoroidal injection was approved by the FDA for the treatment of macular edema associated with uveitis in October. It is the first and only treatment approved for injection into the suprachoroidal space.
Free training on the suprachoroidal injection technique is available through Bausch + Lomb. Ophthalmologists interested in attending a Xipere training session can register online at https://www.xipere.com/hcp/xipere-training.

Newsletter
Keep your retina practice on the forefront—subscribe for expert analysis and emerging trends in retinal disease management.