
Christine Kay, MD, presents new findings on MCO-010, a novel optogenetic therapy improving vision in retinitis pigmentosa patients at the Retina Society meeting.

Christine Kay, MD, presents new findings on MCO-010, a novel optogenetic therapy improving vision in retinitis pigmentosa patients at the Retina Society meeting.
Phase 1/2 interim results highlight real-world functional enhancements in patients with severe LCA5-related Leber congenital amaurosis.

The PRISM clinical trial assesses 4D-150 (4D Molecular Therapeutics) in adults with neovascular age-related macular degeneration, also known as wet AMD.
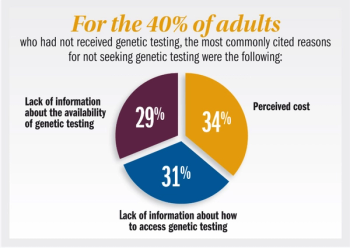
Published: September 28th 2025 | Updated:
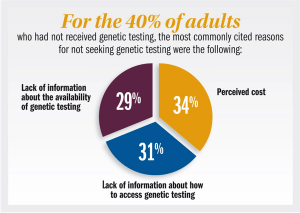
Published: August 27th 2018 | Updated: