Positive data from the GALE long-term extension study showed patients developed fewer new scotomatous points with 36 months of both continuous monthly and every-other-month treatment.

Positive data from the GALE long-term extension study showed patients developed fewer new scotomatous points with 36 months of both continuous monthly and every-other-month treatment.

VISTA is a global randomly assigned, controlled, masked, multi-center pivotal study evaluating the efficacy, safety, and tolerability of 2 dose levels of AGTC-501 for the treatment of X-linked retinitis pigmentosa

Researchers from the Case Western Reserve University School of Medicine and the Cole Eye Institute have found that supplement melatonin could be linked to a decreased risk of age-related macular degeneration.
Injection-free subgroup results demonstrated that a single intravitreal dose of 4D-150 without any supplemental anti-VEGF injections resulted in stable mean visual acuity that was equal to or higher than the standard bimonthly aflibercept control group at all 6 time points through Week 24.
Jennifer Lim, MD, FARVO, spoke with us about her presentation Faricimab vs aflibercept in the reduction of exudates in Y/R trials" at Clinical Trials at the Summit meeting in Park City, Utah on June 8, 2024.
Carl Danzig, MD, spoke with us about his presentation on the 24 week results of the PRISM trials for wet AMD. He gave this presentation at Clinical Trials at the Summit meeting in Park City, Utah on June 8, 2024.
Christina Weng, MD, spoke with us about her presentation on the emerging technology of home OCT and its role in AMD. She gave this presentation at Clinical Trials at the Summit meeting being held in Park City, Utah on June 8, 2024.

Michael Singer, MD, spoke with Modern Retina about his presentation of first time data from the CALM Registry at Clinical Trials at the Summit meeting being held in Park City, Utah on June 8, 2024.

Eleonora Lad, MD, PhD, discusses the stages of dry AMD from early to intermediate to geographic atrophy, while also assessing which endpoints are most useful for these different stages.
Investigators put forth a list of considerations for surgeons ahead of lens removal in pediatric patients
Authors pointed out that understanding this link may aid with genetic counseling or surveillance of affected individuals, potentially contributing to improved management and outcomes.

The primary endpoint for the ReNEW and ReGAIN trials is the rate of change in the macular area of photoreceptor loss assessed by spectral domain-optical coherence tomography (SD-OTC) and ellipsoid zone mapping at week 48.

The IND approval will allow the company to initiate a Phase I/IIa clinical trial for its gene therapy treatment targeting wet Age-related Macular Degeneration (AMD) including Polypoidal Choroidal Vasculopathy (PCV).

The company noted its OCT reference database now offers a library of 870 healthy eyes, more than tripling the previous versions.

Arshad M. Khanani, MD, MA, shares what attendees and the retina community can expect from the Clinical Trials at the Summit meeting being held in Park City, Utah on June 8, 2024.

The electromagnetic driving systems are proposed for the flexible 5-DOF magnetic manipulation of a micro-robot within the posterior eye, enabling precise targeted drug delivery.

Ocugen is developing OCU410 as a 1-time gene therapy for the treatment of geographic atrophy. It utilizes an AAV delivery platform for the retinal delivery of the ROR Related Orphan Receptor A gene.

EyeBio is developing a pipeline of clinical and preclinical candidates for the prevention and treatment of vision loss associated with retinal vascular leakage.

The UME module adds to the list of ophthalmic diseases available in the dataset.

The COVID-19 pandemic changed medicine forever, and now scientists are forging a new path for healthcare in the future, including in ophthalmology.
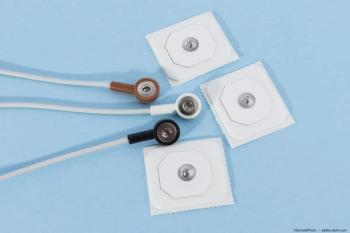
According to researchers at Waseda University in Japan, the proposed device can measure electrical potentials from different places in the retina simultaneously, which is useful in diagnosing eye diseases.

According to the company, its Phase 3 program enrolled 1,984 patients across COAST and ShORe trials. Topline data from both pivotal trials are expected in 2025.
Understanding how the intricate networks of blood vessels in the eye and brain are formed ultimately could inspire new treatments for conditions like diabetic retinopathy and stroke.
Investigators undertook a secondary analysis of the effects of long-term, low-dose aspirin in over 3,000 older adults who satisfied the inclusion criteria in the Aspirin in Reducing Events in the Elderly–AMD (ASPREE-AMD) study.
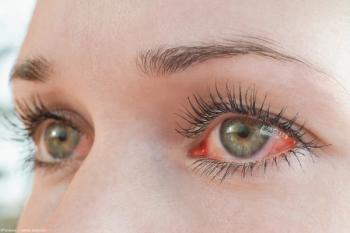
Only symptom in 1 case was conjunctivitis.
Metformin, an anti-diabetes drug, may partially suppress the formation of neovascular tufts on the retinal surface by blocking the mTORC1 signaling pathway

A retrospective study found that the frequency of ocular metastases was relatively low.

The agency approved Yesafili (Biocon Biologics) and Opuviz (Samsung Bioepis, Biogen) as biosimilars to Eylea (Regeneron Pharmaceuticals).

Durga Borkar, MD, MMCi, spoke with Modern Retina about the quality data (Qdata) gathered on geographic atrophy as well as the GATHER 1 and GATHER 2 data she presented at the 2024 Retina World Congress meeting in Fort Lauderdale, Florida.