Few treatment options available for early stages of disease.

The week of September 18-24 will be dedicated to educating the public on IEDs such as uveitis, keratitis, conjunctivitis and more.
With a robust pipeline, new hope for Stargardt disease and retinitis pigmentosa may be on the horizon.
Garg reported the 30-month data showing that patients receiving pegcetacoplan experienced an increasing treatment effect compared with sham (ie, a decrease in GA lesion area over time).
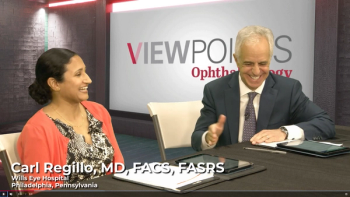
A panel of retina specialists review new and emerging treatments in neovascular AMD and DME, highlighting high-dose aflibercept and KSI-301. Which emerging treatments are you most interested in?

In recent panel discussions, leaders in the retina space shared their thoughts around AMD and DME. These key quotes showcase the heart of these discussions.

When smokers used both cigarettes and e-cigarettes, studies showed additional detrimental findings, such as lower general health, increased sleep latency, and higher vascular disorder risk.

The AI retinal scanning model will be free to use for all institutions.

A panel of retina experts discussed the challenges presented in the management of DME. Which do you think is the biggest of these challenges?

The use of VR mazes hold an intrinsic advantage compared with physical mazes in the ability to control conditions and test various patterns with reliability, participant safety, and repeatability.
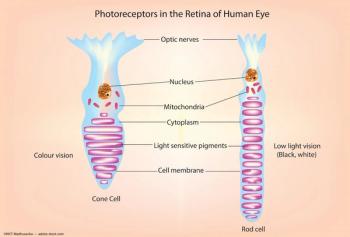
According to the Louisiana State University Health Sciences Center, elovanoids have been shown to restore the structure and integrity of damaged photoreceptor cells by repairing, remodeling and regenerating healthy cells.

A panel of retina specialists weighed in on the dosing intervals when using of faricimab for the treatment of neovascular AMD in the clinical practice setting. Do you agree with them?

The competition is offering an opportunity for amateur and professional photographers to demonstrate their skills and share with the world what they see and what it means to #LoveYourEyes.

According to the company, the clinical study update suggests continued positive trends in best-corrected visual acuity and multi-luminance mobility testing as well as positive trends in low-luminance visual acuity among treated eyes.

A panel of esteemed retina specialists recently delved into innovative therapies for neovascular age-related macular degeneration and diabetic macular edema, focusing on personalized treatment, telemedicine, and the ongoing quest for advancements.

The Ophthalmic World Leaders (OWL) Leadership Summit, scheduled for September 21 and 22, 2023, in Austin, Texas, will feature a lineup of prominent speakers and engaging discussions on a wide range of leadership topics and industry insights.

Novartis acquired Gyroscope Holdings Limited from Syncona in February 2022.

Alex Gorsky currently sits on the boards of Apple, IBM, JPMorgan Chase, and the Travis Manion Foundation, as well as the Wharton School of the University of Pennsylvania Board of Advisors.

The podcast by Cognition Therapeutics features a discussion with retinal specialists.

In this key role, Weil will lead the organization to commercial readiness from designing launch strategies for the US and globally.

Walgreens Boots Allianc Inc. and CVS Health are among the companies the FDA sent letters to for violating federal law.

The company is expanding its patent portfolio to 10 granted patents and 32 pending patent applications.
According to the company, OPGx-LCA5 is designed to address vision loss due to Leber congenital amaurosis associated with mutations in the LCA5 gene, which causes one of the most severe early-onset retinal dystrophies.

The non-profit will partner with the imaging company to prevent blindness and fund new retinoblastoma research.

According to the companies, the collaboration has the potential to benefit patients who have suffered from specific, chronic ocular diseases.

A new survey shows that 95% of adults at risk for certain retinal diseases know a little or nothing about them. Allen hopes her story will help raise awareness and encourage those at risk to regularly prioritize their eye health.

According to the company, 4D-150 comprises its customized and evolved intravitreal vector, R100, and a transgene cassette that expresses both aflibercept and a VEGF-C inhibitory RNAi.

Ocular Therapeutic, Rezolute, and Opthea will all present at the H.C. Wainwright 25th Annual Global Investment Conference being held in New York City in September.

According to the company, it has 9 presentations at the annual congress of the European Society of Cataract and Refractive Surgeons being held in Vienna. AVG also is a sponsor of the International Society of Presbyopia.

The beauty brand announces Ashley Brissette, MD, MSc, FRCSC, will be the brand's first guiding ophthalmologist.